Rickettsial diseases of animals. Laboratory diagnosis of rickettsiosis in farm animals. Manifestations of certain types of disease
Rickettsiosis is a group of acute vector-borne infectious diseases caused by rickettsia and characterized by the development of generalized vasculitis, intoxication, damage to the central nervous system, and specific skin rashes. This group does not include bartonellosis (benign lymphoreticulosis, Carrion disease, bacillary angiomatosis, bacillary purpuric hepatitis) and ehrlichiosis (Sennetsu fever, monocytic and granulocytic ehrlichiosis).
ICD-10 code
A75 Typhus
A79 Other rickettsial diseases
Epidemiology of rickettsial diseases
All rickettsial diseases are divided into anthroponoses (typhus, relapsing typhus) and natural focal zoonoses (other infections caused by rickettsia). In the latter case, the source of infection is small rodents, cattle and other animals, and the carrier is blood-sucking arthropods (ticks, fleas and lice).
Rickettsial diseases are widespread diseases recorded on all continents. In developing countries they account for 15-25% of all febrile illnesses of unknown etiology.
Pathogenesis of rickettsioses
Penetrating through the skin, rickettsia multiply at the site of penetration. In some rickettsioses, a local inflammatory reaction occurs with the formation of a primary affect. Then hematogenous dissemination of the pathogen occurs, as a result of which generalized warty vasculitis develops (skin rashes, damage to the heart, membranes and substance of the brain with the formation of an infectious-toxic syndrome).
Symptoms of rickettsial diseases
In most modern classifications, three groups of rickettsial diseases are distinguished.
- Typhus group:
- epidemic typhus and its recurrent form - Brill's disease (anthroponosis, causative agent - Rickettsia prowazekii Rocha-Lima, carriers - lice);
- epidemic (rat) typhus (pathogen Rickettsia mooseri, pathogen reservoir - rats and mice, carriers - fleas);
- Tsutsugamushi fever, or Japanese river fever (causative agent - Rickettsia tsutsugamuchi, reservoir - rodents and ticks, carriers - ticks).
- Group of spotted fevers:
- Rocky Mountain spotted fever (caused by Rickettsia rickettsii, reservoir - animals and birds, carriers - ticks);
- Marseilles, or Mediterranean, fever (pathogen - Rickettsia conori, reservoir - ticks and dogs, carriers - ticks);
- Australian tick-borne rickettsiosis, or North Australian tick-borne typhus (causative agent - Rickettsia australis, reservoir - small animals, carriers - ticks);
- tick-borne typhus of North Asia (pathogen - Rickettsia sibirica, reservoir - rodents and ticks, carriers - ticks);
- vesicular, or smallpox, rickettsiosis (pathogen - Rickettsia acari reservoir - mice, carriers - ticks).
- Other rickettsial diseases: Q fever (pathogen - Coxiella burneti, reservoir - many species of wild and domestic animals, ticks, vectors - ticks).
Diagnosis of rickettsial diseases
Clinical diagnosis of rickettsial diseases
All human rickettsioses are acute cyclic diseases (with the exception of Q fever, in which a chronic course is possible) with severe intoxication, characteristic symptoms of damage to the vascular and central nervous system, and typical exanthema (except for Q fever). Each rickettsiosis is characterized by a specific clinical picture. Thus, symptoms of tick-borne rickettsiosis occur on the 6-10th day after a tick bite and include the appearance of a primary affect at the site of tick suction, which is a typical inoculum scab (“tache noir”), and regional lymphadenitis.
Laboratory diagnosis of rickettsial infections
Laboratory diagnosis of rickettsiosis consists of identifying the pathogen and specific antibodies.
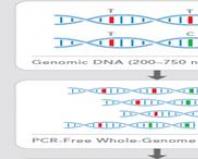
Isolation of the pathogen is an absolute diagnostic criterion. Rickettsiae are grown in tissue cell cultures. They are isolated primarily from blood, biopsy samples (preferably from the area of inoculation of the scab) or mite biomass. Work with rickettsia is allowed only in specially equipped laboratories that have a high degree of protection, so isolation of the pathogen is rarely carried out (usually for scientific purposes).
Rickettsial infections are diagnosed using serological methods: RIGA, RSK with rickettsial antigens, RIF and RNIF, which allows you to separately determine IgM and IgG. Microimmunofluorescence is considered a reference method. ELISA, which is used to identify the pathogen, determine its antigens and specific antibodies, has become widespread.
Until now, Weil-Felix RA is used, based on the fact that the blood serum of patients with rickettsiosis is capable of agglutinating AC strains, OX2, and OX3, Proteus vulgaris.
x
x
(Rickettsiosis)
a group of infectious diseases of humans and animals caused by rickettsiae (See Rickettsiae) : characterized by spread through blood-sucking arthropods - carriers of infection.
Rickettsial infections in humans. These include: epidemic, or louse-borne, typhus and its recurrent form - Brill's disease (carriers are lice); endemic, or flea (rat) typhus (reservoir of the causative agent - rats and mice, carriers - fleas): Marseilles, or Mediterranean fever (reservoir - ticks and dogs, carriers - ticks): tick-borne R., or tick-borne typhus of the North Asia (reservoir - rodents, ticks, vectors - ticks): North Australian tick-borne typhus (reservoir - small animals, vectors - ticks); smallpox and vesicular R. (reservoir - mice, carriers - ticks); tsutsugamushi fever, or tsutsugamushi, or Japanese river fever (reservoir - rodents and ticks, carriers - ticks); Q fever (reservoir - many species of wild and domestic animals and ticks, carriers - mainly ticks); trench (Volyn trench), or five-day fever (reservoir - humans, carrier - body louse): tick-borne paroxysmal R. (reservoir - rodents, carriers - ticks). Of these, louse-borne and flea-borne typhus, trench and Q fever, tick-borne vesicular and paroxysmal R. were recorded on the territory of the USSR at various times.
R. infection occurs through tick bites or when infected feces of lice and fleas get into wounds (scratching) and mucous membranes. In some cases (Q fever), R. spreads through the secretions of sick animals (urine, feces, milk). The reservoir of infection for R. (except for typhus and trench fever) is animals, for the most part wild (especially rodents), in which the infection is usually asymptomatic. Blood-sucking vectors become infected from infected animals. In addition, the reservoir of infection in nature for many R. is ticks, in which transovarial transmission (from generation to generation) of rickettsia is possible. The presence of a reservoir of infection in nature determines the natural focality (See Natural focality) of most R. In some R. (for example, louse-borne typhus), the source of infection is a person.
In humans, R. occurs in the form of febrile diseases of varying severity with a variety of symptoms; some R. are accompanied by a characteristic rash. Flea typhus (caused by Muser's rickettsia) occurs when infected flea feces come into contact with damaged skin (scratch marks); incubation period from 5 to 15 days; a characteristic symptom is a bright pink rash on the skin not only of the torso and limbs, but also of the face, appearing on the 4th-5th days diseases; the course is milder than with louse-borne typhus. With vesicular R., the incubation period is 1-2 week; a week before the onset of fever, a compaction appears at the site of the tick bite with a vesicle in the center, which is subsequently covered with a black scab and surrounded by a zone of hyperemia: the elements of the rash dry out and form dark crusts. With paroxysmal R., the incubation period is 7-10 days; relapses of fever are typical; Induration at the site of the tick bite and rash are usually absent. See also Typhus , Quintan , Q fever , Marseille fever , Tsutsugamushi .
For laboratory diagnosis of R., serological methods are used (agglutination, hemagglutination, complement fixation reactions, etc.). In some cases, a bacteriological study is carried out. The main method of treatment for R. is antibiotics. Prevention of R. - control of vectors, for example, lice in typhus, disinfestation, use of repellents (See Repellents) , protective (against tick attacks) suits, veterinary and sanitary restrictions on the use of milk from sick people and meat from sick and forcedly slaughtered animals. For some R. (typhus, Q fever), active immunization is used .
Lit.: Zdrodovsky P.F., Golinevich E.M., The doctrine of rickettsia and rickettsiosis, 3rd ed., M., 1972.
V. L. Vasilevsky.
Rickettsia in animals. In veterinary practice, the most common infectious hydropericarditis (coudriosis), Q fever, rickettsial keratoconjunctivitis and rickettsial monocytosis (erlichiosis). Infectious hydropericarditis affects cattle and pigs. First described in 1838 by F. Trigardt in South Africa. Pathogens: Cowdria ruminantium (in ruminants) and C. suis (in pigs). The source of the infectious agent is sick and recovered animals; carriers are ixodid ticks. The disease is manifested by high fever, disturbances in the functioning of the heart and breathing, diarrhea, convulsions, and in acute cases usually ends in the death of animals. A characteristic pathological sign is the accumulation of exudate in the pericardium and body cavities. No specific treatment has been developed. Prevention: isolation of sick animals, destruction of ticks, vaccinations. Rickettsial keratoconjunctivitis occurs in cattle, camels, pigs, poultry. First described in 1931 in South Africa (D. W. A. Coles). The causative agent is Ricolesia bovis. The source of the infectious agent is sick animals; transmission route is airborne. The disease is characterized by swelling of the eyelids, damage to the conjunctiva, photophobia and a benign course (on the 8th-10th days animals recover). Treatment: solutions of collargol, zinc sulfate, antibiotic ointments. Prevention: isolation of patients, disinfection of premises. Rickettsial monocytosis affects cattle and dogs. First described in 1935 in Algeria. Pathogens in cattle: Rickettsia bovis, R. ovina; in dogs R. canis. The source of the infectious agent is sick animals, the reservoir is pasture ticks. The disease manifests itself as fever and more often ends in the recovery of animals that are long time become carriers of rickettsia. A characteristic feature is the detection of rickettsiae in monocytes. Treatment: sulfonamides. Prevention: isolation of patients, destruction of ticks, disinfection of premises.
Lit.: Epizootology, under general. ed. R. F. Sosova, M., 1969.
42 43 44 45 46 47 48 49 ..2. ANIMAL DISEASES, CAUSED BY RICKETSIA(RICKETSIOSES)
2.1. GENERAL CHARACTERISTICS OF RICKETSIA AND RICKETSIOSES
According to modern taxonomy and nomenclature of bacteria, the order Rickettsiales includes three families: Rickettsiaceae, Bartonellaceae and Anaplasmataceae. The order was named after the American microbiologist X. Ricketts (1871-1910).
Based on the morphology of pathogens, adaptability to existence in the cells of arthropods and mammals, as well as some other characteristics, the family Rickettsiaceae is divided into three tribes, of which Rickettsiae itself includes three genera: Rickettsia, Rochalimea and Coxiella.
Most representatives of the genus Rickettsia live in obligate intracellular associations with eukaryotic hosts (vertebrates or arthropods). Some types of rickettsia cause diseases in humans (typhoid fever, Rocky Mountain spotted fever, tsugamushi fever, etc.) or other vertebrates (rickettsial keratoconjunctivitis) and invertebrates. According to the morphology of rickettsia, they are pleomorphic microorganisms of coccoid (0.3...0.4 µm), rod-shaped (up to 2.5 µm), bacillary or filamentous forms. Often form diplo forms. They have a three-layer cell wall, which is typical for gram-negative bacteria. As a rule, they are motionless. They are stained with basic aniline dyes, according to Romanovsky-Giemsa and others. They reproduce by binary fission in the cytoplasm or simultaneously in the cytoplasm and nucleus of certain cells of vertebrates and arthropods. Grows well in chicken embryo cell culture and in some mammalian cell lines. Aerobes form hemolysin and produce toxic substances similar to bacterial toxins that are not released into the environment. The optimal temperature for growth is 32...35°C.
Rickettsia are weakly resistant to external environment, quickly die at high temperatures and under the influence of ordinary disinfectants. They are resistant to low temperatures (they retain virulence for a long time in a lyophilized state at -20...-70 °C). They are resistant to sulfonamide drugs and sensitive to tetracycline antibiotics.
Coxiella are similar to representatives of the genus Rickettsia, but unlike them, they reproduce in vacuoles (phagolysosomes) of host cells, and not in the cytoplasm or nucleus. The genus includes one species, Coxiella burnetii, which causes Q fever in humans and animals. C. burnetii are polymorphic short rods (0.2...0.4x0.4...1 µm), gram-negative, without capsules, immobile. They reproduce only in vacuoles (phagolysosomes) of host cells. Cultivated in the yolk sac of a chicken embryo, they are resistant to heating up to 65 ° C and the action of chemicals.
The tribe Erlichiae includes three genera: Erlichiae, Cowdria and Neorickettsia.
The genus Cowdria includes one species - C. raminantium, the causative agent of cowdriosis (hydropericarditis) in ruminants. Morphologically, coudria are pleomorphic coccoid or ellipsoidal (0.2...0.5 µm), less often rod-shaped cells (0.2...0.3x0.4...0.5 µm), gram-negative, immobile. They are localized in the vacuoles of the cytoplasm of ruminant vascular endothelial cells, where specific compact colonies are formed. According to Giemsa they are dyed dark blue and accept other aniline dyes well. They do not grow on artificial nutrient media. Transferred ixodid ticks genus Amblyomma. Sensitive to sulfa drugs and tetracycline.
Caused by rickettsiae, they are increasingly being diagnosed in humans of different ages, infection mainly occurs through transmission after infection. One of the reasons for the increase in statistical indicators is the popularity of tourism, however, you can also become infected at home, because carriers of pathogens love to live in gardens, on wet grassy lawns, and in sheds.
Clinical prognosis of the disease is favorable provided timely diagnosis and treatment.
Rickettsial diseases are transmissible febrile diseases caused by rickettsiae.
Rickettsia can live in the body of rodents or cattle, and the most common carriers of infection are head lice, body lice, and ticks. These pathogenic microorganisms enter the human body through the skin.
Tick-borne rickettsiosis is caused by pathogens that are in salivary glands ah ticks. Survival of rickettsiae under conditions environment very low, but they can persist at low temperatures or drying.
There are several types of rickettsiosis (you can learn more about them below), but they are all united by similar characteristics (clinical, immunological, pathogenetic, etc.).
Penetrating into the human body, rickettsia cause inflammation of the lymph nodes and also enter the blood, leading to rickettsia and toxemia.
Types and groups of disease
Rickettsial diseases are divided into 2 groups:
- anthroponotic (pathogens are carried by body lice and head lice, the source of the disease is a person infected with rickettsia);
- zoonotic (transmitted by tick bites, the source of infection is rodents and small livestock).

The term “rickettsiosis” refers to 6 groups of diseases caused by rickettsia:
- (epidemic and endemic);
- a group of tick-borne fevers (Rocky Mountain spotted fever, tick-borne typhus of North Asia, Marseilles or Mediterranean fever);
- Tsutsugamushi fever;
- Q fever;
- paroxysmal rickettsiosis (tick-borne paroxysmal rickettsiosis and trench fever);
- rickettsioses of animals.
Each type of this disease has its own pathogen.
Depending on the symptoms, human rickettsioses are divided into groups:
- typhus group (epidemic typhus, Tsutsugamushi fever);
- group of spotted fevers (Rocky Mountain spotted fever, Marseilles fever, smallpox rickettsiosis, tick-borne typhus of North Asia);
- other rickettsial diseases, including Q fever.

Signs of typhus
Routes of human infection
There are several ways of infection with rickettsiosis pathogens:
- transmissible - transmission through saliva blood-sucking insect(most common for tick-borne rickettsiosis);
- contact - through interaction with objects “contaminated” with rickettsia;
- blood transfusion - during blood transfusion;
- aspiration – entry of pathogens onto mucous membranes respiratory tract;
- transplacental – infection of the fetus from the mother;
- nutritional – with food or liquid contaminated with waste products of a sick animal.
Aspiration is the least common method of transmission.
Symptoms of tick-borne rickettsioses
At the first stages, tick-borne rickettsiosis most often has nonspecific symptoms; over time, more striking symptoms appear:
- fever (body temperature can reach 40 degrees);
- muscle pain;
- severe headaches;
- pain in the joints;
- body aches, general weakness;
- decreased appetite;
- nausea and vomiting;
- cardiac dysfunction (tachycardia or bradycardia);
- pain in the intestinal area;
- pain in the area of the lymph nodes.

These symptoms are typical for rickettsioses transmitted by tick bites; individual signs depend on the type of disease. Let us consider the manifestations of the most common types of rickettsial diseases in Russia.
Manifestations of certain types of disease
Signs of tick-borne rickettsiosis (tick-borne typhus):
- bright pink rashes on the skin;
- severe headaches;
- weakness in the body;
- increase in body temperature.
Symptoms of Marseilles fever:
- hyperthermia of the mucous membrane of the oropharynx, sore throat;
- gray coating on the tongue;
- at the site of the bite - tissue necrosis, the formation of a black or brown scab;
- swollen lymph nodes;
- rash (appears 2-3 days after the bite), gradually affecting the entire body;
- The rashes are first spotty, then macular-popular, and can take on the appearance of red pimples.
- After the rash subsides, pigment spots remain on the skin.

Smallpox rickettsiosis (pathogens are transmitted by the bites of gamasid ticks) makes itself felt by a number of signs.
- Infiltrate. A non-itching red infiltrate of the skin from 5 to 20 mm at the site of the bite, which after a few days turns into a vesicle, it breaks through and becomes covered with a black scab.
- Rash. Papulo-vesicular rash over the entire body except the soles and palms (as in smallpox).
- Scarring. After the rash disappears, shallow scars remain, which smooth out after at least 3 weeks.
- Relapse. Repeated erythematous (manifested by redness and swelling) or maculopapular rash 2-3 days after the bite, later it turns into blisters. The secondary rash does not leave scars.
- Fever. Appears repeatedly (recurs).
Due to vesicular rash, this type Rickettsiosis is sometimes called vesicular. Smallpox rickettsiosis is easily confused with chickenpox, but when infected with rickettsia, the blisters are deeper and denser, and the rash affects the entire body at once.
Rocky Mountain spotted fever is one of the most dangerous species rickettsial diseases, since without treatment it can lead to death. Its symptoms are:
- chills followed by fever;
- nosebleeds;
- convulsions;
- deterioration of vision and hearing;
- disturbance of consciousness

The listed types of rickettsioses can occur in mild, moderate or severe form.
Diagnostics
If tick-borne rickettsiosis is suspected, contact an infectious disease specialist.
Diagnosis of the disease begins with an analysis of the primary affect on the human body (local inflammatory reaction to the bite) and the symptoms of rickettsiosis, which allows us to draw preliminary conclusions about the type of disease.
- collection of epidemiological anamnesis;
- serological methods (RIF, ELISA, RIGA and RSK) are used to isolate rickettsia from the patient’s blood;
- linked immunosorbent assay;
- Weill-Felix agglutination reaction;
- general blood test (if infected, there is a decrease in the concentration of leukocytes and lymphocytes in the blood and an increase in ESR);
- laboratory tests of urine, cerebrospinal fluid;
- Allergy skin tests help with differential diagnosis.

During diagnosis, the similarity of the course of rickettsiosis with the following diseases is taken into account:
- flu;
- smallpox;
- measles;
- hemorrhagic fevers;
- enterovirus infection;
- severe allergies;
- meningococcal infection.
Treatment of the disease
Treatment of rickettsial infections is carried out conservatively. Antibiotics of the tetracycline group are prescribed:
- tetracycline (1.2 -2 grams per day, divided into 4 doses),

- doxycycline (100-200 grams per day in a single dose).

Levomycetin and fluoroquinolones are also often prescribed.
The drugs depend on the type of rickettsiosis. The course of treatment is established based on the febrile period + 2-3 days after stabilization of the patient’s condition and normalization of body temperature.
Treatment with drugs is complex, therefore, in addition to antibiotics, anti-inflammatory drugs are prescribed medicines and medications for detoxification and desensitization therapy.
Tick-borne rickettsiosis in severe form requires treatment with corticosteroid hormonal agents.
In addition to these methods of therapy, there is symptomatic treatment, for example, skin treatment (removal of dead areas of skin, scabs), which is required by smallpox rickettsiosis or intravenous administration electrolyte solutions for prolonged fever.
All employees of a medical institution are required to wear protective suits and follow safety rules during medical procedures to avoid the spread of infection.
Mild forms of the disease can be treated at home, following the prescription of a specialist.
Preventive measures
Prevention of rickettsiosis is carried out in three directions:
- individual human safety;
- veterinary measures;
- agrotechnical methods.
Personal protective measures:
- avoiding contact with rodents;
- protection against ticks (special suits, thick clothing, tick repellents, Pavlovsky nets, inspection of human skin after walks in nature);
- compliance with personal hygiene rules;
- compliance with sanitary standards.
Note! If a tick is found on the skin, it must be immediately removed and taken to laboratory test to the sanitary and epidemiological service.
Veterinary and agrotechnical methods are aimed at combating rodents, ticks and protecting food and water from contamination by animal waste products.
The term "rickettsia" was proposed in 1916 by the founder of the doctrine of rickettsia and rickettsiosis, the Brazilian scientist E. da Roja Lima in honor of the American pathologist G.T. Ricketts, who was the first to discover the causative agent of spotted fever in the blood of patients in 1909 Rocky Mountains and has proven the role of ticks in transmitting this disease. G. Ricketts died in Mexico City from typhus while studying it. A great contribution to the study of rickettsial diseases was made by the Czech microbiologist S. Provacek, who also died from typhus while studying the disease in Serbia. The role of lice in the transmission of infection during typhus was first established in 1908 by N.F. Gamaleya. The most important role in the development of the doctrine of rickettsial diseases and in the creation of a classification of rickettsial diseases was played by the works of P.F. Zdrodovsky and his students.
Epidemiology. Rickettsial diseases are found in all countries of the world. Two of them - epidemic typhus and Volyn fever - are epidemic anthroponoses. They are characterized by the fact that the source of infection is a sick person or carrier, and the carrier is a body or head louse, in which rickettsia causes a fatal infection.
By the nature of transmission, all rickettsioses are vector-borne diseases. Only the causative agent of Q fever, although sometimes reserved by ticks, however, due to its high resistance in the environment, can also be transmitted by contact, nutrition and airborne droplets.
Infected ixodid and gamasid ticks secrete rickettsiae mainly from the infected salivary glands directly to the bite site. In lice and fleas, rickettsiae multiply in the cells of the intestinal wall and are excreted in the feces on the skin around the bite. Infected lice develop a disease that ends in their death. In fleas and ticks, the infection is asymptomatic. Ticks transmit the pathogen transovarially.
Many researchers believe that rickettsiae became parasites of arthropods in ancient times and that the evolution of rickettsioses went from tick-borne rickettsioses with natural focality to rat rickettsioses with transmission of infection by fleas and, finally, to epidemic louse-borne typhus.
Of the zoonotic rickettsioses, the most important have Q fever, tick-borne rickettsiosis, rat typhus, tsutsugamushi fever. Foci of rat typhus and Marseilles fever exist in certain areas of the Black Sea and Caspian coasts. Vesicular rickettsiosis is found only in the central regions of Ukraine. Natural foci of tsutsugamushi fever have been identified in the Primorsky and Khabarovsk territories, Kamchatka and Tajikistan. However, the incidence of these rickettsioses is low. Among the anthroponotic rickettsioses, Volyn fever entered the nomenclature of infectious diseases during the First World War; it was also noted during the Second World War. Of primary importance is louse-borne typhus, which is a “companion of wars and social upheaval.”
Etiology. For a long time it was believed that rickettsiae are microorganisms that, in evolutionary and biological terms, occupy an intermediate position between bacteria and viruses. It has now been established that rickettsiae are gram-negative bacteria that, having adapted to intracellular existence, have retained their own enzyme systems that ensure the autonomous metabolism of these microorganisms, but have lost the ability to withstand adverse environmental influences. Therefore, when exposed to conditions of extracellular existence, rickettsia die.
The most important feature of the life activity of rickettsia in the body is their ability to produce toxic substances of a protein nature - endotoxin associated with the rickettsial membrane. Endotoxin acts on tissues by uncoupling oxidative phosphorylation.
There are non-pathogenic (42 species) and pathogenic (more than 30 varieties) rickettsiae. Non-pathogenic rickettsia live in arthropods and do not cause disease in humans or animals. Pathogenic rickettsia They live in arthropods and cause specific diseases in mammals, including humans.
Classification of rickettsia and rickettsioses. Rickettsia is represented by 3 genera: Rickettsia, Coxiella and Rochalimaea. The most numerous is the genus Rickettsia, whose representatives cause three main groups of rickettsioses.
1. Group of tick-borne spotted fevers (the most ancient
group). Includes tick-borne rickettsiosis(tick-borne typhus
North Asia), Marseilles fever, vesicular ricketts
siosis, Rocky Mountain fever and tick-borne typhus
nogo Cleveland. The rickettsiae that cause these diseases are characterized by
are characterized by the presence of common antigens. They are also characterized by
signs of ecological community are natural focal in-
infections, the reservoir of which is Ixodidae (gamasaceae)
ticks, as well as wild and domestic animals.
2. Group of lice-flea typhus. It includes
2 genetically and serologically similar, but ecologically and epi-
Demiologically different diseases:
a) anthroponosis - epidemic, or absorbed, typhus;
b) zoonosis - endemic, or rat (flea) rash
Noah typhus.
It is believed that louse-borne typhus is the result of the adaptation of rat typhus rickettsia to the human body and to a new carrier - lice. IN last years This group includes a rare disease, the causative agent of which R. Canada has the properties of rickettsia of the typhus group and tick-borne spotted fever (the causative agent was isolated in Canada from ixodid ticks).
3. Pathogens of this group cause a number of diseases. Tsutsugamushi, or Japanese river fever, causes R. tsutsugamushi. The source and carrier are ticks (transovarial transmission of rickettsiae).
Q fever(coxiellosis) is a zoonosis of domestic and wild animals caused by Coxiella.
The pathogen of the genus Rochalimaea, unlike rickettsia and cocciella, grows on artificial nutrient media and causes an anthroponotic disease - Volyn trench fever, or six day fever carried by lice.
Pathogenesis and clinical and morphological manifestations. They are characteristic of rickettsial infections. Entrance gate It is usually the skin at the site of an insect bite, where an infectious agent is rubbed in along with feces, which then spreads hematogenously.
The pathological process in human rickettsioses is due to the fact that rickettsia, with the exception of Coxiella (Q fever), multiply mainly in the endothelium of the capillaries, which leads to the development of granulomatous vasculitis, often accompanied by thrombosis. The latter, in combination with the vasoparalytic effect of rickettsial endotoxin, causes significant disturbances in the central nervous system and circulatory disorders. Clinically, all human rickettsioses are acute diseases with severe intoxication, often a typhoid state, a characteristic symptom complex of damage to the central nervous system and cardiovascular system, and the presence of a characteristic exanthema (with the exception of Q fever). Moreover, each rickettsiosis has its own fairly typical clinical picture. With Q fever, a chronic course of the process is possible. Epidemic typhus, Rocky Mountain spotted fever, and Tsutsugamushi fever are severe diseases with a high mortality rate, which reached 50% before the use of antibiotics. After suffering rickettsial infections, persistent immunity usually remains.
EPIDEMICSYPNOYTIF
. Epidemic typhus(typhus exanthematicus) is an acute febrile rickettsial disease characterized by damage to small vessels of the brain, toxicosis, and widespread roseola-petechial rash.
The disease is also known as “European”, “historical”, “cosmopolitan”, “louse typhus”, “military”, “famine typhus”, “hospital fever”. All these numerous synonyms indicate that typhus accompanies a person during periods of social upheaval, disasters, and wars. Typhus is an ancient infection, but it was identified as a separate nosological form only in early XIX century. It is believed that epidemic typhus already occurred in ancient Greece. Several major typhus epidemics have been described in the Middle Ages.
From 1805 to 1814/g. All of Europe was covered in typhus. The spread/infections were of a severe pandemic nature. A particularly disastrous situation arose in the French army during its retreat from Russia: in Vilna, out of 30,000 French prisoners of war, 25,000 died of typhus. Large epidemics of the disease among troops of both sides were observed during the Russian-Turkish and especially the Crimean (1854-1855) campaigns.
Even during times of relative calm, typhus was noted in all provinces of Russia, and as soon as the population suffered hunger and poverty, the incidence of typhus increased again.
Typhus acquired a threatening character during the years of the civil war of 1918-1920, when, according to L.M. Tarasevich, 20 million people fell ill with typhus.
There was an increase in the incidence of typhus in the second world war. In the current decade, the incidence of typhus is sporadic. According to statistical data, the share of typhus among infectious diseases is 0.07%.
Etiology. The causative agent of the disease is Rickettsia Provacek. In the epidemiological aspect, typhus is a true anthroponosis. The source of infection is a sick person, starting from the last 2-3 days of the incubation period, the entire febrile period and until the 7-8th day from the moment the body temperature normalizes - about 20 days in total. The possibility of long-term carriage is allowed, and therefore repeated, so-called endogenous, incidence may occur. Transmission of the infection occurs from sick people to healthy people transmissibly through body lice, mainly body lice - Pediculus vestimenti, and to a lesser extent through head lice - Pediculus capitis, in which rickettsiae, which enter the stomach when sucking, cause fatal rickettsiosis with destruction of the epithelium of the gastric mucosa and the entry of a huge amount of rickettsia into the lumen of the gastrointestinal tract. Infection of a person occurs by scratching the skin wound formed after a bite and rubbing infected louse feces into it.
Susceptibility to typhus is universal. However, during epidemic outbreaks, the majority of patients are aged 18-40 years.
Since lice are the only link in the general epidemiological chain of typhus, then from the development of lice and partly from biological properties Lice depend on a special pattern of epidemics of this disease: the incidence of typhus begins to increase in the fall and reaches a peak in February-April. During these months, optimal temperature conditions are created for the development of lice. main reason winter-spring rise in incidence - seasonal deterioration of sanitary and hygienic conditions.
Sporadic cases of epidemic typhus, occurring during the inter-epidemic period and often eluding the medical and sanitary service, when infested with lice, can be a link between the end of the previous local epidemic and the beginning of the next one.
Immunity. The disease leaves behind a stable, although not absolute, immunity. There are indications of cases of repeated and even triple infection with typhus. The nature of the immunity acquired after typhus is two-profile - anti-infectious and anti-toxic. Anti-infectious immunity begins to form following infection and persists for 10-25 years. There is a point of view about the unsterility of immunity in rickettsial diseases and, in particular, in typhus. According to this point of view, the pathogen is not completely destroyed, but is in a “dormant” state, which supports immunity and protects against superinfection. Only with the disappearance of rickettsia from the body does immunity cease.
Rickettsia enters the human body through damaged skin and, as experiments show, end up in the blood within 15 minutes. Part of the rickettsia dies under the influence of bactericidal factors, and part, due to tropism, is adsorbed on the surface of the endothelium, mainly capillaries and precapillaries, in which slow blood flow and the smallest lumen of blood vessels contribute to best contact rickettsia with cells. Rickettsia are phagocytosed by the endothelium, where they multiply with the subsequent formation of Muser cells - cells whose cytoplasm is filled with rickettsia. Rickettsia multiply most intensively during the incubation period (10-12 days) and 1-2 days of the febrile period. In response to the introduction and reproduction of the pathogen, swelling and desquamation of the endothelium occurs, which is destroyed with the release of rickettsia into the blood. The process of introduction of rickettsia into new cells and their reproduction is repeated many times until the amount of the pathogen reaches a certain threshold value, causing massive rickettsia. Partial death of rickettsia is accompanied by toxinemia, the threshold degree of which marks the onset of the disease - a febrile period.
The trigger and main mechanism in the development of the pathological process is the angioparalytic effect of rickettsial endotoxin. A generalized toxic-paralytic lesion of the microvasculature occurs, especially capillaries and precapillaries, with an increase in their permeability, plasmorrhagia, which is accompanied by a decrease in the volume of circulating blood. In paralytically dilated capillaries, blood flow slows down, followed by the formation of blood clots, which leads to hypoxia and dystrophic changes in the internal organs. These changes are especially pronounced in the medulla oblongata, which leads to irritation of the vasomotor center and a drop in blood pressure. These phenomena intensify from the 6th to 8th day of the disease, when, as a result of the penetration of small vessels into the endothelium and the proliferation of rickettsia in it, generalized vasculitis develops with predominant damage to the central nervous system, especially the medulla oblongata and skin. At the height of the febrile period (2-3 weeks of illness), swallowing disorders and dysphagia (boulevard phenomena) may develop due to damage to the medulla oblongata. Widespread vasculitis in combination with disorders of nervous trophism reduces the stability of tissues: the patient easily develops tissue necrosis and bedsores. Damage to the sympathetic division of the autonomic nervous system and the adrenal glands increases arterial hypertension and is accompanied by impaired cardiac activity, which can lead to death.
The main changes in typhus are detected only microscopically. When autopsying a person who died of typhus, the diagnosis can only be made tentatively. Traces of a rash in the form of brown and red spots and dots are found on the skin. Particularly characteristic is the presence of a conjunctival rash, which is constantly observed in the 2-4th week of the disease. The brain substance is full-blooded, soft, the soft meninges are dull (serous meningitis), the spleen is enlarged (its weight is 300-500 g), soft, full-blooded, its tissue yields a small scraping of the pulp on the incision. Dystrophic changes are noted in other organs.
Microscopic examination of organs, especially the central nervous system and skin, reveals changes in capillaries and arterioles characteristic of typhus vasculitis. These changes were studied in detail by L.V. Popov, N.I. Ivanovsky, I.V. Davydovsky, Sh.N. Krinitsky, A.I. Abrikosov, A.P. Avtsyn. Initially, swelling, destruction, desquamation of the endothelium and the formation of blood clots (parietal or occlusive) are observed. Then the proliferation of endothelium, adventitial and perithelial cells increases, lymphocytes and individual neutrophils appear around the vessels, and focal necrosis develops in the vessel wall. Changes in blood vessels can vary both in intensity and in the degree of participation of proliferative, necrobiotic or thrombotic processes. Based on this, several types of typhus vasculitis are distinguished: warty endovascular disease, proliferative vasculitis, necrotizing vasculitis. You can often talk about typhus destructive-proliferative endothrombo-vasculitis. It should be noted that foci of endo- or perivascular infiltration have the form of nodules, which were first discovered in typhus by L.V. Popov (1875). Subsequently, the nodules were recognized as the most characteristic formations of typhus and were called Popov’s typhus granulomas.
Typhoid granulomas are found in all systems and organs, with the exception of the liver, spleen, lymph nodes and bone marrow, but the structure of granulomas and the nature of vasculitis are different in different organs. In the brain, granulomas are surrounded by a wide zone of proliferating microglial cells. In the skin, endo- and perithelia of capillaries and adventitial cells of arterioles and venules, as well as lymphoid cells surrounding the vessel and single neutrophils, take part in the formation of granulomas. The lumen of the vessel in the center of the formed granuloma, both in the brain and in the skin, is difficult to recognize or is completely lost in the mass of proliferating cells. In the sympathetic department of the autonomic nervous system, typhus granulomas form in the same way as in the brain.
In 90% of cases, a characteristic exanthema occurs in the skin. Typhoid rash (exanthema) appears on the skin on the 3rd-5th day of the febrile period of the disease. Morphologically, it is characterized by the previously described changes in the vessels of the microcirculatory bed and small arteries with the formation of granulomas. If necrotizing vasculitis predominates, hemorrhages (petechiae) may appear in the skin, which is usually observed in severe typhus.
In the brain, typhus nodules usually form in the 2nd week of the disease and disappear at the beginning of the 6th week. They are found in the pons and peduncles of the brain, subcortical nodes, medulla oblongata (especially often at the level of the inferior olives), and the posterior lobe of the pituitary gland. There are no nodules in the white matter of the cerebral hemispheres. In addition, hyperemia, stasis, perivascular (mainly perivenous) couplings of plasma and lymphoid cells, and focal proliferation of microglia are observed in the brain tissue. Alternative changes in nerve cells do not reach a large extent. Based on these changes, we can talk about the development of typhus encephalitis, which goes with serous meningitis. These changes in the central nervous system lead to disorders of the patient’s consciousness and psyche, which are combined into the concept of a typhoid state (status typhosus), so characteristic of typhus.
In the sympathetic section of the autonomic nervous system and its ganglia, inflammatory changes develop with the formation of granulomas and infiltrates of lymphoid cells, hyperemia; nerve cells undergo significant changes - there is typhus ganglionitis. Inflammatory changes are also found in the peripheral nervous system - neuritis.
The heart is constantly affected in typhus, which is expressed by the development of dystrophic changes in the myocardium or interstitial myocarditis, which manifests itself in focal, less often diffuse infiltration of the stroma with plasma cells, lymphocytes, and the formation of granulomas. The severity of myocarditis may vary.
Arteries of large, medium and small caliber in typhus are often involved in the process: necrosis of the endothelium, sometimes segmental necrosis of the muscular membrane is observed, which leads to parietal or obstructive thrombosis and the development of local hemodynamic disorders - gangrene of the extremities, foci necrosis in the brain, retina.
Complications typhus are diverse and are caused by changes in blood vessels and the nervous system. Trophic disturbances often develop. In the skin, from slight pressure, foci of necrosis appear on protruding areas of the skin and bedsores. When the secretion of the salivary glands is suppressed due to damage to the cervical sympathetic ganglia, conditions are created for the development secondary infection: Purulent parotitis and otitis media develop, ending in sepsis. With subcutaneous injections of drugs, foci of necrosis of the subcutaneous tissue (fiber) appear - oleogranulomas (fat necrosis can also occur spontaneously). As a result of circulatory disorders (vasculitis) and due to weakening of the heart (myocarditis), bronchitis and pneumonia develop. Complications of typhus during epidemic outbreaks vary both in frequency and nature. During the Great Patriotic War complications were observed in 30% of patients with typhus. The most common of them were pneumonia, bedsores, purulent parotitis, and subcutaneous tissue abscess.
Death in typhus it occurs due to heart failure (about 70% of cases) or complications.
In the past, typhus was accompanied by high mortality, which in some epidemics reached 60-80%. The highest mortality rate was observed in people over 40 years of age. Typhus in children is mild and has low mortality.
DISEASEBRILLA (SPORADICSYPNOYTIF)
. Brill's disease (syn.: sporadic typhus, repeated typhus, recurrent typhus, Brill-Zinsser disease, etc.) - repeated (or late endogenous relapse) typhus due to activation of Provacek's rickettsiae, which remained in a latent state in in the body of persons who have previously suffered from typhus.
Epidemiologically, the disease is characterized by sporadicity, and clinically - by a benign, mild course with preservation of the main features of epidemic typhus.
History of the study And geographical distribution. In 1898, N.E.Brill in New York amid an epidemic typhoid fever observed cases of benign febrile illness similar to mild form typhus. In 1934, H. Zinsser, after studying materials about 538 patients who had immigrated to the United States from Europe, put forward the hypothesis that this disease was a relapse of epidemic typhus suffered many years ago. Subsequently, this assumption was confirmed in the works of many scientists. The International Classification of Diseases, adopted at the 19th World Health Assembly, allows for a double name for the disease - Brill disease and Brill-Zinsser disease. After the Second World War, this disease was observed in many European countries, Australia, and South Africa. In our country, Brill's disease has been registered since 1958.
Epidemiology. The source of infection is a sick person. If they have lice, patients with Brill's disease can serve as a source of epidemic typhus.
Features of the epidemiology of modern typhus, which in 60-100% of cases is represented by Brill's disease, are sporadic, lack of lice, focality and seasonality characteristic of epidemic typhus. The disease is registered both in places of former epidemics and in territories free from typhus, among persons arriving from areas unfavorable for it. Sporadic typhus affects mainly elderly and senile people who have survived epidemics of this infection.
Etiology. The causative agent of the disease is Rickettsia Provaceca, which is similar in morphological, biological, antigenic and other properties to classical strains. Laboratory studies and clinical observations of patients infected through lice from repeatedly ill people, in whom the primary disease with typhus was much more severe than that observed with Brill's disease, refute the assumption of reduced virulence of the pathogen the last one. The milder course of Brill's disease is explained by the presence of residual immunity in those who are repeatedly ill after previously suffering from typhus.
Pathogenesis and pathological anatomy. It is believed that the occurrence of Brill's disease is due to the activation of Provacek's rickettsia, which remain latent in the human body for a long time after suffering epidemic typhus. Based on clinical and experimental studies, it is suggested that during latent typhus infection, Provaceca rickettsia are located in sedentary (tissue) macrophages—stellate reticuloendotheliocytes, lung macrophages, histiocytes of the peritoneum and skin, which have less bactericidal activity: in These rickettsiae are protected from the action of specific antibodies, and their localization directly in the cytoplasm, and not in phagocytic vacuoles, avoids contact with lysosomes. A latent infection can flare up as a result of exposure of the body to sharp temperature fluctuations(cooling), surgical interventions, shock, various injuries, infectious diseases, etc. The pathogenesis of the disease is not qualitatively different from that of epidemic typhus, but the process is less pronounced. Characterized by corresponding vascular damage, the presence of Popov's granulomas and the vasodilating effect of rickettsia toxin. Granulomas are detected, although in smaller numbers than with typhus, in the brain, skin, adrenal glands, myocardium and mucous membranes. The concentration of the pathogen in the blood in Brill's disease is less than in epidemic typhus, so its isolation is difficult.
Complications with Brill's disease are observed in 5.3-14 % cases. Most often it is pneumonia. Thromboembolic complications usually occur in older people.
Forecast. As a rule, favorable, mortality is 0.5-1.7%. Elderly and senile people with an unfavorable premorbid background die more often.
The rarity of complications, absence or low mortality, and rapid convalescence distinguish Brill's disease from epidemic typhus.
KU- FEVER
. Q fever- a unique zoonotic rickettsial disease characterized by severe fever, general intoxication syndrome and damage to various organs and systems (lungs, liver, nervous system, etc.).
Q fever is the only representative of the group of pneumotropic rickettsioses (pneumorickettsioses). Many consider the term “pneumorickettsiosis” to be unfortunate due to the fact that the disease is extremely polymorphic in its manifestations, and lung damage is not the only symptom.
History of study and geographical distribution. Q fever was first reported in 1933 in Australia among slaughterhouse workers. E.N. Derrick (1937) called it Q-fever after the first letter of the English word “query,” which means “unclear.” Before World War II, cases of Q fever occurred only in Australia and occasionally in the United States. But during the war, there were large outbreaks of the disease (“Balkan flu”) among British and American troops fighting in the Balkans and Italy. In the post-war years, this disease was identified in all countries.
Etiology. The causative agent of Q fever is Burnet's rickettsia, or Coxiella burneti. Characteristic feature Burnet's rickettsia is their high resistance to various physical and chemical agents. They survive in dry tick feces for up to 586 days, and in dried urine and blood of animals for up to 6 months. Rickettsia tolerates both high and low temperatures well.
Pathogenesis. Burnet's rickettsiae are very highly infectious. They penetrate the body through the mucous membranes of the respiratory tract and digestive tract, through the conjunctiva, damaged and even intact skin, which leads to a variety of methods of infection: - aspiration (air-dust) - the most typical; people working on livestock farms, meat slaughterhouses, leather and wool processing enterprises, as well as those living near them near roads along which livestock are driven, become infected; - airborne infection can occur during lambing and calving of livestock, when rickettsia with tiny droplets of blood, mucus, etc. are inhaled by people; this way you can become infected from a person with Cu-rickettsial pneumonia; - the nutritional route is possible when consuming contaminated foods and water;
The contact path is carried out in contact with contaminated material;
Transmission through tick bites is rare; Obviously, ticks are important in the preservation and spread of rickettsiosis among animals and birds in natural foci and in the transmission of infection to domestic animals.
Susceptibility to Q fever is high. Representatives of all age groups of the population are affected. At the same time, professional groups of the population associated with farm animals (animal technicians, veterinarians, cattlemen, shepherds, milkmaids, shepherds, grooms, etc.) are more often affected. Children are often infected, mainly through nutritional means, but their disease is mild or asymptomatic, leaving strong immunity. Persons who arrived in endemic areas contract Q fever in the first 3-5 years of living in them.
Complications. Relatively rare and observed only in severe cases of the disease. Thrombophlebitis, pancreatitis, pyelonephritis, epididymitis, pleurisy, pulmonary infarction, meningo-encephalitis have been described. They are often caused by the addition of a secondary infection. Currently, thanks to properly administered therapy, complications are practically not observed.
Forecast. Q fever is one of the relatively benign rickettsioses. With it, recovery almost always occurs, although the period of complete recovery in some patients is somewhat longer than with other rickettsial diseases. Among the numerous descriptions of the disease, only isolated cases of death have been recorded.