Low-melting solders for soldering. DIY hard silver solder
- What is silver solder?
- Chemical composition of the alloy for joining materials
- How to cook silver solder using the old method
- Melting silver solder
- Properties of medium melting alloys
- How to properly prepare solder at home?
To save money, it is advisable to make silver solder yourself, although this step can only be done by those who have certain knowledge. You can learn how to solder using various solders purchased at retail stores.
If you are unsure of your abilities, you can buy solder rather than making it yourself.
What is silver solder?
Pure silver is an expensive metal and is rarely used for work. Its structure is soft, so craftsmen use alloys that include copper, zinc, as well as aluminum, nickel, and cadmium.
Compounds of silver with copper and zinc have high melting properties and are used as silver solder. The soldering seam made with this solder is very durable. Solder with 10% silver can be easily processed with a hammer in rollers and is used for soldering steel parts.

PSR-25 solder is used for joining brass surfaces.
PSR-25 and PSR-12 solder is used to connect brass surfaces and make especially neat and clean soldering areas. The silver solder form is a solid sheet that is cut to form strips. required size. For work, strips with a thickness of 1.5-2 mm are used, and small parts connected using strips 3 mm wide. Silver solder is used to fill gaps between seams. They withstand significant vibration loads, are resistant to shock and deformation.
The composition of silver solder is established by GOST, which regulates the area of its use.
Return to contents
Chemical composition of the alloy for joining materials
Modern technological processes soldering involves the use of silver solder, which, when connecting nodes, is suitable for step soldering. Such work requires the use of an alloy that can withstand temperatures of 600°C.
The solder contains ingredients such as 30% silver, 20% copper, 16% zinc, 33% cadmium. The alloy is very brittle and is intended for soldering materials that are not subject to vibration. The composition with the amount of silver increased to 52% is very fluid, but withstands loads well during multi-stage stages of soldering materials.
Return to contents
How to cook silver solder using the old method
There are several ways to create a silver alloy, but you can master the technique perfectly only after long practice. To obtain the alloy, you need to prepare 2 coins: a fifty-kopeck piece from 1924 and a nickel from 1962. You will also need:

It is most convenient to heat solder on a gas burner.
- silver;
- spoon (not tablespoon);
- ingus;
- gas-burner.
At the beginning of the work, silver is melted in a spoon. Add a five-kopeck coin to the resulting melt and roll the mixture over a spoon for better mixing. The longer the spoon is rolled, the better the mixing process occurs. But there is one significant drawback: Many components needed for solder burn out.
Then the master who makes the alloy with his own hands pours it into ingus and rolls it out without annealing. The resulting solder is of high quality: 10 g refers to the 900th sample.
When soldering, it is very important to prepare fresh flux, which will ensure high-quality work. It is necessary to carefully monitor the size of the flame in the burner: a soft, not very hot fire in the form of a broom will ensure a high-quality seam.
For work, hard solders are used, which contain: 80 Ag, 16 Cu, 4 Zn, 75 Ag, 22 Cu, 3 Zn. Soft solders include: 65 Ag, 20 Cu, 15 Zn.
For getting lung silver solder used in repairing products must be prepared: 7 parts silver, 2.8 parts brass, 0.35 parts zinc. It is important to clean the brass from the oxide film before starting work. To obtain 10 g of solder, 999 silver is melted. After receiving liquid composition add brass, mix the contents in a spoon. When the composition has completely melted, add zinc, shake the spoon several times, then begin to roll the resulting composition. After its manufacture, it is necessary to cut the rolled sheet using scissors and weigh it on a scale. For successful work should be prepared:
- sandpaper;
- spoon;
- gas burner;
- mixing spatula;
- folds;
- scissors;
- scales.
An amateur craftsman cannot do without such materials as:

Pure silver is not used for solder, as it is too expensive a metal.
- silver;
- brass;
- pure zinc;
- borax, which is added to the molten composition.
Sometimes pure zinc is not added: it is better to use its alloy with brass or copper. Zinc is added to the solder, wrapped in silver foil.
To make silver solder, you can take 96th standard silver and burn 94th standard. It is obtained by burning old silver products: brocade, braids, galloons.
Return to contents
Melting silver solder
By melting the metals that make up the alloy in a crucible, silver solder is obtained. The crucible is placed in a furnace or the components are melted using a blowpipe. Before work you need to prepare the equipment:
- crucible;
- wooden stick or iron hook;
- borax;
- charcoal mixture;
- container with water.
Solder components are melted using borax. The order of the operation is observed: refractory metals are melted first, then low-melting metals are added.

The solder components are melted using borax.
To make your own solder in a crucible liquid metals constantly interfere with a wooden rod or an iron hook. To create solder more High Quality the whole process is divided into two stages. The crucible is pulled out of the furnace, and the metal is combined in a container with water. Fine-grained drops are formed, which are dried and melted a second time, covered with borax on top.
After complete melting, the metal is poured into molds. The frozen tiles or bars are rolled into strips, which are crushed on a lathe.
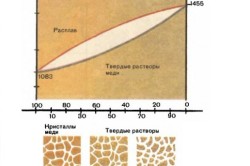
For technical soldering of silver objects, alloys consisting of metals are suitable: 20% copper + 80% silver, 4% tin + 48% brass. When soldering steel silver solder Consider the phase diagram between copper and silver.
The simplest refractory solder is pure copper. As is known, copper has relatively high strength and at the same time great ductility; in the cast state, copper has a tensile strength of 17-18 kg/mm2 with an elongation of 30-35%; the tensile strength of deformed copper, depending on the amount of impurities and the degree of hardening, varies within 24-25 kg/mm2; The elongation of high-purity copper reaches 50%. In this regard, connections soldered with copper have high strength and are not prone to brittle fracture.
Pure copper has the highest electrical conductivity after silver, so soldering with copper is desirable for connections that should not create additional resistance to the passage of electric current.
Copper well wets the surface of iron and steels (including stainless and heat-resistant), nickel and nickel alloys, metal-ceramic hard alloys, etc. This property of copper makes it possible to widely use it as a refractory solder; however, the high melting point of copper (1083°) makes the soldering process technologically difficult, so pure copper is used as solder only when soldering in furnaces (with a protective atmosphere).
For soldering gas burner It is not advisable to use copper, since the water vapor generated in this case (as a result of the reduction of copper oxide with hydrogen) can cause cracks in the seam.
Copper-zinc solders. As can be seen from the phase diagram, the addition of zinc to copper causes a decrease in the melting point of the alloy; increasing the zinc content in the alloy to 40% reduces the melting point to 900°. Such an alloy can be used as a solder not only for soldering steel, but also for metals with a lower melting point, such as copper.
Copper-zinc alloys containing up to 36-39% Zn are, in equilibrium, a homogeneous solid solution having the same crystal lattice, like copper itself; these alloys have high ductility. Alloys containing from 39 to 46% Zn have a two-phase structure consisting of crystallites; alloys of this composition are less ductile, and the greater the relative amount of phase, the higher the brittleness of the alloy.
An increase in the zinc content in an alloy from 40 to 50% leads to the disappearance of crystals, and alloys of this composition have a single-phase structure consisting of crystallites. Alloys with 50-59% Zn have a two-phase structure, which, when the zinc content increases above 59%, again transforms into a single-phase structure of homogeneous y-crystallites, which have low mechanical strength and very little elongation.
The mechanical properties of copper-zinc alloys are completely dependent on the chemical composition. From the copper-zinc system, a fairly wide group of alloys containing from 30 to 65% zinc are used as solders. According to the domestic standard, copper-zinc solders are produced in three compositions. Copper-zinc solders are usually produced in the form of grains ranging in size from 0.2 to 5.0 mm.
Comparing chemical composition copper-zinc solders with a diagram of the dependence of mechanical properties on the composition of the alloys, it can be seen that all solders of this class, and especially PMC-36, have great fragility and insufficient strength. Therefore, such solders are not particularly widespread in industry.
PMC-36 solder is used only when you need inexpensive brazing solder with a possibly low melting point, for example for soldering L-62 brass. PMC-48 solder is suitable for soldering copper alloys with a melting point above 900-920°, and then only if the soldered joint is not subject to shock loads, vibration and bending.
PMC-54 solder is intended for soldering copper, bronze and steel, for products that do not experience impact and bending loads. In the case when from solder connection higher strength and mainly good resistance to impact and bending are required; L-62 and L-68 brasses are often used as solder. These alloys, along with a higher tensile strength (approximately 30 kg/mm2), have a very high elongation, reaching 40%. Connections soldered with brass can undergo significant deformation without failure. Brasses can be used for soldering copper, steel, nickel and cast iron.
When soldering with ordinary brass, zinc inevitably burns out, which, as is known, already boils at 906°. Burnout of zinc is accompanied by the formation of zinc oxide vapors, which are very harmful to workers, and severe slagging of the solder. In addition, a decrease in zinc content during burnout leads to an increase in the melting temperature of the solder, which in turn requires an undesirable increase in the soldering temperature. In order to somewhat weaken these disadvantages of brasses, small additives of tin and silicon are sometimes introduced into their composition.
The addition of tin slightly lowers the melting point of brass and increases its fluidity. The addition of silicon reduces the burnout of zinc, since when brass is melted, silicon first of all oxidizes itself and, combining with flux, forms a dense film of borosilicates that protects zinc from evaporation. As a result of this, solders on copper base, containing, in addition to zinc, small amounts of tin and silicon, have better technological properties and provide more high density and seam tightness.
The addition of tin and silicon to brass causes a change in the microstructure and properties. The introduction of silicon into L-62 brass leads to a decrease in the amount of a-phase in the structure; with the introduction of 0.6% Si, the a-phase completely disappears and only one b-phase remains. The ductility of L-62 brass increases with an increase in the silicon content in it to 6%, while the tensile and shear strength does not decrease; Alloys containing approximately 0.3% Si have the greatest strength. Despite the fact that silicon somewhat impairs the spread of molten brass over the metal surface, the strength of the weld is still higher. Tin, unlike silicon, improves the spreadability of brass and the ability to fill solder seams.
It is known to use copper-zinc solders in industry, improved by nickel, which increases the strength and reliability of solder joints. However, the melting point of such solders is significantly higher than that of nickel-free brass.

Solders K1, KZ, PSr ZKd of the Cd-Ag system provide heat resistance of copper solder joints up to a temperature of 250 °C (short-term). The most heat-resistant compounds (up to 300 °C) from copper and brass can be obtained by soldering with solders of the Cd-Ag-Zn system (PSrbKTs and PSrBKTsN). The higher heat resistance of copper joints soldered with these solders, compared to the heat resistance of the solders themselves, is probably due to the alloying of the seam with copper, which was transferred to the seam during soldering. PSr5KTs and PSr8KTsN solders have satisfactory ductility in the cast state.
Cadmium solders are characterized by a higher temporary resistance (o>110-200 MPa) than tin- and lead-based solders (18.6 - 42.1 MPa). The high strength of cadmium solders is not realized in solder joints made of copper and brass due to the formation in them of a low-plastic layer of intermetallic compounds, along which premature destruction of the solder joint occurs. The microhardness of the light phase (intermetallic compound) is equal to the microhardness of brass; the amount of intermetallic in the seam increases with the duration of the soldering process, i.e., the time of contact of liquid solder with copper or copper alloys. In this case, increasing embrittlement of the soldered seam is observed.
Steels are soldered with cadmium solders only after they have been copper-plated. Activation of cadmium solders with zinc, which has a high chemical affinity with iron, made it possible to use them for soldering steels and at the same time increase their strength. Solder of this type, containing 60-85% Cd, 15-50% Zn and 0.4-5% Ni with a melting point of 290-270 ° C, is suitable for soldering not only copper, zinc and brass, but also steels, including including corrosion-resistant. The yield strength of butt joints made of copper sheet 2 mm thick, soldered with such solder, is 228.3 MPa; Meanwhile, the temporary tensile strength of connections made of the same metal, soldered with tin-lead solder, is 53.9 MPa. This solder does not contain silver and is used for soldering products in the electrical industry and heat exchangers. The introduction of nickel into the solder additionally activates and strengthens it, since nickel forms a continuous series of solid solutions with iron, and with cadmium - a phase like y-brass.
There is information that the introduction of sodium Cd-Zn into solders (2-5%) gives it the properties of self-fluxing and heterogeneity. Typical composition of solder proposed by O. P. Ksenofontov: 10-20% Zn; 2.5% Ni; 0-3% Ag; Cd - the rest.
Additional strengthening of solder Cd—(10–40% Zn) is possible by adding 0.0001–0.3% Ca and (or) Mg to it. These additives also increase the heat resistance of the solder and improve its spreadability. Tensile strength of butt joints made of low-carbon steel soldered with this solder is 248.9-253.8 MPa (with solder without these additives 210.7 MPa).The tensile strength of joints at a temperature of 200 °C is 40.2-42.6 MPa, whereas for joints soldered with solder without calcium and magnesium additives, under the same conditions it is equal to 28.1 MPa. Corrosion tests of soldered joints for 500 hours in a 3% solution of sodium chloride showed a slight decrease in their strength.
High mechanical properties and good wetting ability of Cd-(10-40)% Zn solders, according to Iwanaga Singichiro, can be achieved by introducing titanium (0.05-0.5%) or copper and titanium (0.05-1%) into them . This solder is suitable for soldering complex-shaped products made of low-carbon steel or copper. The addition of silver to cadmium solders in quantities that do not cause the formation of brittle phase inclusions in the weld ensures high strength and ductility of the solder joint.
According to A. M. Robertson and others, for soldering composite materials matrix based aluminum alloy and boron fiber filler, Cd-5% Ag solder turned out to be suitable. The shear resistance of the joints at a temperature of 20 °C is 83.3 MPa; Maximum temperature operation 315 °C.
Cadmium solders (Cd—25% Sn), which have low electrical resistance, are used in the installation of computers and calculating machines.
Zinc solders
Zinc, among other fusible metals (tin, lead, cadmium), has the most high temperature melting (419 °C).
When alloying zinc with cadmium, tin, and aluminum, the melting point decreases due to the formation of low-melting eutectics. The melting onset temperature decreases most significantly when zinc is alloyed with tin (199 °C); the eutectic Zn - Cd melts at 266 °C, and the eutectic Zn - A1 at 382 °C.
When silver or copper is added to zinc, the melting point of zinc alloys increases due to the formation of peritectics. Currently, some zinc alloys with aluminum, cadmium, copper, silver, tin, and lead have been studied and used as solders, the melting temperature of which is in the range of 340–480 °C.
Zinc solders have a number of features that determine their use. The vast majority of zinc-based solders are characterized by relatively low ductility, low strength and poor ability to spread and flow into the gap.
Alloying zinc with tin, aluminum, and cadmium not only leads to a decrease in the temperature of the beginning and end of solder solidification, but also significantly affects their mechanical properties. For example, among the Zn-Sn alloys, the most durable and sufficiently ductile alloys are those containing 20-30% Sn. However, these alloys have a large crystallization range (199–375 °C) and, what is especially important, low temperature solidus and therefore are not promising for soldering joints operating under heating conditions up to temperatures of 200-250 ° C. K. K. Hardy showed that the relative elongation of zinc alloys with tin (20-25% Sn) largely depends on the cooling rate during solidification. The relative elongation of the alloy cast in a mold heated to a temperature of 200 °C is 5.2%.
Solders of the Zn-Cd system are characterized by very low ductility, even when the cadmium content in them reaches 40% (P300A). Zinc-aluminium alloys, which are close in composition to the eutectic Zn - 5% Al (melt = 380 °C), are also low-plasticity.
The ductility of zinc-based solders alloyed with aluminum and joints soldered by them can be slightly increased by introducing 1-5% Al into them; in this case, the melting temperature of the alloy increases by approximately 20 °C (PSr5KTsN solder). Zinc alloys with small amounts of copper are relatively ductile (<3 %). Их прокатывают в фольгу. Технологические характеристики цинковых припоев существенно зависят от состава паяемого металла.
The phase diagram of copper and zinc has a relatively flat liquidus line. In this regard, zinc solders in the liquid state cause intensive development of chemical erosion of copper and its alloys during the soldering process; in this case, the ductility of the weld metal sharply decreases. The most appropriate method is soldering with these solders using high-frequency heating, electrical contact method, etc. When soldering with zinc solders, the heat resistance of soldered copper joints is less than when soldering with cadmium solders. Zinc forms chemical compounds with iron; When soldering steels with zinc solders, layers of such compounds are formed along the border with the seam.
One of the ways to prevent the formation of intermetallic layers along the boundary of the soldered metal and solder as a result of their chemical interaction is to alloy the solder with elements that have a greater chemical affinity for the soldered metal than the solder base. Such elements when soldering steel with zinc include aluminum. Therefore, all zinc solders intended for soldering steel or iron are currently alloyed with small amounts of aluminum.
Zinc solders with cadmium, aluminum and copper are most often used for soldering aluminum alloys (Table 12). Their most important advantage is the relative fusibility and good corrosion resistance of the joints they solder, especially those soldered with zinc solders alloyed with aluminum and copper.

Zinc and aluminum form a eutectic and a wide range of solid solutions. To reduce the erosive effect on aluminum alloys, zinc solders are alloyed with elements that reduce their melting point and have a low limiting solubility of aluminum at soldering temperatures. Such elements include, for example, tin and lead. However, lead, unlike tin, which forms a eutectic with zinc, chemically interacts weakly with zinc (phase diagram with monotectic).
Solder for soldering copper must be a pure metal or alloy with a lower melting point than the parts it joins. During soldering, it, being in a molten state, fills the gap between the parts being soldered, and after hardening, it holds them together. Solder, made from pure metal, turns into a liquid state at a very specific temperature, and alloys usually soften gradually, over a certain temperature range.
Copper is a metal with a low melting point, which makes it easy to solder.
In order for the adhesion of the soldered parts to be of high quality, the straightened solder must spread over their surfaces and “wet” them. Fluxes are used to remove oxide films and other contaminants that impede wetting. The widespread connection of parts using soldering is due to a number of advantages of this process:
- preservation of the shape and size of the parts being soldered, since they themselves do not melt;
- a connection can be obtained without warping and noticeable internal stresses;
- connection strength and process performance;
- the initial temperature of the parts being soldered has virtually no effect on the quality of the process;
- the possibility of connecting not only metals in various combinations, but also metals with non-metals;
- in most cases, soldered parts can be desoldered if necessary.
Soldering copper and its features
Copper products lend themselves very well to soldering. The fact is that copper is a chemically low-active metal; even when heated and melted, it reacts weakly with oxygen contained in the air and other chemically active substances. That is why it can be relatively easily cleaned of oxides and contaminants without using aggressive and complex fluxes.
In addition, there are quite a lot of metals and alloys with low melting points that perfectly wet copper in the molten state. Thanks to this, almost any type of soldering can be done with copper parts, using a very large number of different solders. It is possible to obtain soldered seams with a very wide range of properties. It is not for nothing that more than 97% of all soldering in the world is carried out to connect together parts made of copper or alloys of which it is the basis.
Return to contents
Solder for soldering copper
The physical properties of a soldered joint and its reliability are largely determined by the metal or alloy on which it was created in this case. All solders used for copper soldering are divided into two types:

Solders for copper must be selected based on their composition and melting point.
- Low-temperature, which melt at temperatures of no more than 450 ° C. The strength of the seam created by such soldering is relatively low, but, thanks to the relatively low temperature, the physical properties of the parts being soldered, especially their strength, do not change.
- Solders that have a higher melting point are considered high-temperature. The strength of the seam with this soldering is higher, but there is a possibility of a decrease in the strength of the parts being soldered as a result of their annealing.
As for their chemical composition, the following types are most often used:
- tin, lead and lead-tin;
- tin-copper, tin-silver and tin-copper-silver;
- copper-silver-zinc and copper-phosphorus;
- silver
Solders from the first group are low-temperature and are most often used for soldering electronic circuits. They are usually used in everyday life to repair various metal products. When making printed circuits, cadmium or bismuth is often added to the alloy to lower the melting point.
The rest of the listed metals and alloys are most often used at home to connect pipelines made of copper. For their reliable, durable and easy joining with each other, a capillary joining technique has been developed, which can be used at both low and high temperatures.
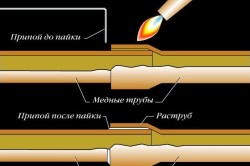
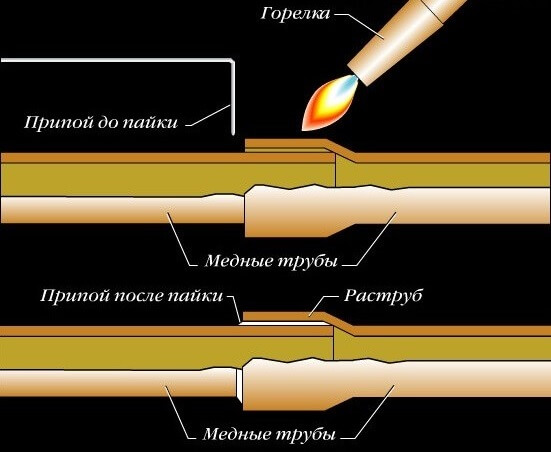
When a pipe is inserted into a fitting, the gap remaining between them is no more than 0.4 mm wide. Due to this, a capillary effect occurs during soldering: molten solder fills this entire space. This happens because the interaction forces between the molecules of copper and the molten metal are greater than between the molecules of the melt itself.
Thanks to this effect, the melt almost instantly fills the entire gap between the pipe and the fitting, and the resulting connection is strong and reliable. You just need to make sure that the surfaces to be joined are well cleaned beforehand and the appropriate fluxes are used.
Since lead is toxic, alloys containing it cannot be used for the installation of drinking water pipelines. In fact, the connection of copper pipelines is carried out using only four types of solders:
- Tin-copper (S-SN97Cu3) and tin-silver (S-Sn97Ag5) are low-temperature. The connection is strong and resistant to corrosion.
- Copper-silver-zinc L-Ag44 (silver - 44%, copper - 30% and zinc - 26%) is high-temperature. The connection is strong, ductile, corrosion-resistant, and has increased thermal conductivity.
- Copper-phosphorus CP203 (copper - 94% and phosphorus - 6%) are high-temperature and can be used without fluxes. The seam is strong, but its elasticity decreases at low temperatures.
- High-temperature silver solder produces a strong, ductile, and corrosion-resistant weld, but it is expensive. Flux is required when soldering.
The list of metals and alloys used for copper soldering is not limited to this. There are quite a lot of them, but they are practically not used at home.
Today, copper pipes have a wide range of applications. In order for the connections to be made reliably and the pipeline to operate smoothly for many years, it is best to solder such products. Unlike other metals, copper can be soldered very well.
The surface of the pipes is thoroughly cleaned of dirt and oxides. When soldering copper, a capillary effect occurs, which results in wetting of the surface. In this case, the alloy disperses over the entire surface and reliably connects the parts.
Modern soldering methods
The main operational characteristics of copper products are regulated in accordance with GOST. Copper is an excellent option for plumbing, heating, gas, and air conditioning systems. The positive characteristics of copper pipes include:
- High anti-corrosion properties;
- Non-toxic;
- Bactericidal properties;
- High resistance to ultraviolet radiation;
- Reliability;
- Strength;
- Durability.
Today, copper pipes are manufactured in varying degrees of hardness. They are:
- Soft;
- Solid;
- Semi-solid.

Soft products are used for plumbing and heating systems, while hard and semi-solid products are used for those pipelines where high mechanical strength is needed.
The only disadvantage of copper is its high price compared to other materials. In order to carry out high-quality installation of plumbing or heating, copper products must be soldered.
Soldering is a permanent connection of pipes using molten material - solder, whose melting point is lower than the material of the parts being connected.
If the correct soldering technology is followed, the connections are very strong and reliable. According to GOST, there are the following types of soldering:
- High temperature;
- Low temperature.
High-temperature soldering is carried out at temperatures above 450˚C and is used for pipes with heavy loads. This type of soldering provides high strength seams due to the strength of solid materials. During low-temperature soldering, the temperature reaches 450˚C.
Low-temperature soldering is carried out with an electric soldering iron, and high-temperature soldering is carried out using a gas torch.
Distinctive characteristics of soldering materials
Depending on the soldering method, the solder can be soft or hard. The following metals are used as soft alloys:
- Lead;
- Tin.

Hard metals include:
- Copper;
- Zinc;
- Silver;
- Phosphorus.
Copper can be soldered with both soft and hard alloys. Soft alloy is used to connect water supply systems. The material for soldering parts is produced in coils in the form of wire with a diameter of 2-3 mm. The carbide is manufactured in the form of profile rods. Hard solder is used to connect those parts where a particularly strong connection is required at high temperatures. Hard solders are used:
- In the manufacture of various instruments;
- For connecting pipelines;
- When carrying out vehicle repairs;
- For carrying out repair work on household products.
Copper plays a very important role in the formation of strong seams during high-temperature soldering. Copper solder is a component of almost any solid metal, and in most cases it is their basis. Phosphorus alloy for soldering consists of copper with phosphorus. Phosphorus alloy is widely used in the refrigeration industry.
The three-component alloy containing silver differs:
- High corrosion resistance;
- Durability;
- Plasticity.

Zinc solder is mostly used for aluminum materials. Zinc alloy has the following advantages:
- Fusibility;
- Corrosion resistance.
Depending on the percentage of the metal composition, the melting point changes. The more zinc contained, the lower the melting point.
The silver alloy provides very strong and tight seams. It has a low melting point and is characterized by the following properties:
- High strength;
- Plasticity;
- Impact resistance;
- Anti-corrosion;
Silver solder can be used to solder any metal. But due to the fact that silver is an expensive material, it is used in cases where a particularly high-quality connection is required.
Correct technology for performing work
According to the requirements regulated by SNiP, soldering with solid materials is necessary when repairing refrigeration equipment or air conditioning systems.
Soft solder is used to connect communications.
In order to connect copper pipes, it is necessary to prepare the following materials:
- Solder;
- Flux;
- Pipe expander;
- Gas burner;
- Soldering iron;
- A brush.
The use of flux is very important. It is intended for:
- Cleaning the surfaces of parts from oxides;
- Better spreading of the alloy;
- Protecting compounds from oxygen.
When soldering copper, fluxes are used, which are regulated by GOST. These fluxes contain pure borax components. Fluxes are available in liquid or powder form.
When low-temperature soldering, it is convenient to use special construction hair dryers, which help heat the pipes from all sides.
Today, manufacturers offer various options for gas burners for copper products:
- Professional;
- Semi-professional;
- For heating pipes.

The connection of pipes is carried out in a consistent manner and in compliance with the following rules:
- The surface of the pipes is cleaned of dirt and oxides with a brush;
- The parts are adjusted to each other and coated with flux;
- Apply solder and secure with soft wire;
- The parts are heated to melt the solder;
- The parts are cooled slowly.
With high-temperature solder, it is correct when it melts from the heat of the heated connection, and not from the flame of the burner.
When performing work, it is necessary to strictly adhere to all safety standards, since when exposed to high temperatures, dangerous vapors are formed with alloys, which can lead to burns. To comply with safety measures you must:
- Wear special acid-resistant clothing;
- Use protective gloves to avoid burns;
- Carry out work in a well-ventilated area.
By observing all the above rules, soldering copper using an alloy will be performed efficiently and reliably, and the work will be carried out without harm to human health.




