Brazing. Brazing alloys
Brazing
TO category:
Soldering
Brazing
Brazing is used to produce strong and heat-resistant seams.
Brazing is carried out observing the following basic rules:
– as with soft soldering, the surfaces are adjusted to each other by soldering, thoroughly cleaned of dirt, oxides and fats by mechanical or chemically;
– the fitted parts at the junction are coated with flux, pieces of solder (copper plates) are placed on the junction and secured with soft knitting wire;
– prepared parts (blanks) are heated with a blowtorch, in a forge or electric furnace;
– when the solder melts, remove the part from the heat and hold it in such a position that the solder cannot flow from the seam;
– then the part is slowly cooled. It is impossible to cool parts with a soldered plate in water, as this will weaken the strength of the connection.
Another soldering method is used: the prepared part (product) is heated and sprinkled with borax, then heated and the end of a copper or brass wire is brought to the joint, which, melting, fills the junction. As they cool, the soldered parts are washed in water, wiped with dry rags and dried; The seam is cleaned with sandpaper or filed with a file.
Soldering defects, their causes and preventive measures are as follows:
solder does not wet the surface of the metal being soldered due to insufficient flux activity, the presence of an oxide film, grease and other contaminants. To prevent non-wetting, fluoride salts are added to the flux or its quantity is increased, improving the processing of parts by removing traces of corrosion and grease; solder sagging or dripping due to insufficient heating of the part; the solder has not melted.
Rice. 1. Tinning of parts: a - immersion in a bath of tin, b - heating of parts for tinning, c - maintenance by rubbing tin
Occupational safety when performing soldering and tinning. Workstations intended for soldering work small parts, must be equipped with local exhaust devices, ensuring air movement speed directly at the soldering site of at least 0.6 m/s.
In rooms where soldering work was carried out, the floors must be washed; dry cleaning of the floor is not permitted. Storing clothing in rooms where soldering is carried out is prohibited.
In the immediate vicinity of workplaces intended for soldering small parts with soft solders, the following should be installed: a washbasin, a tank with a 1% solution acetic acid for pre-washing hands and easy-to-clean portable containers for collecting paper or cotton napkins and rags. There should always be soap, brushes, and napkins for wiping hands near the sink. Using towels common use not allowed.
The preparation of metals and the soldering process are associated with the release of dust, harmful fumes of non-ferrous metals and salts, which, when entering the human body through the respiratory organs, esophagus or skin, cause irritation of the mucous membrane of the eyes, skin damage and poisoning.
Therefore, when soldering and tinning, the following rules must be observed;
The soldering workplace must be equipped with local ventilation;
work in gas-polluted areas is not allowed;
after finishing work and before eating, wash your hands thoroughly with soap;
Add chemicals carefully in small portions, avoiding splashes.
Acid getting into the eyes can cause blindness; acid fumes are very harmful;
store sulfuric acid in glass bottles with ground stoppers in wicker baskets with soft lining;
Use only diluted acid. When diluting, the acid should be poured into the water in a thin stream, continuously stirring the solution. It is forbidden to pour water into acid, since when water combines with acid, strong chemical reaction with the release of a large amount of heat. Even when a small amount of water enters the acid, the water quickly heats up and turns into steam, which can lead to an explosion; – manual operations in which direct contact of the worker’s skin (washing, grinding in products, bottling, etc.) with dichloroethane (a flammable toxic liquid) or mixtures containing it are not allowed; – when heating the soldering iron, observe general rules safe handling with a heating source; – when working with blowtorches: check the serviceability of the lamp, pour fuel into the lamp no more than 75% of the capacity; It is unacceptable to add or pour fuel into a lamp that has not cooled down; Fill a kerosene lamp only with kerosene; work electric soldering iron, the handle of which must be dry and non-conductive.
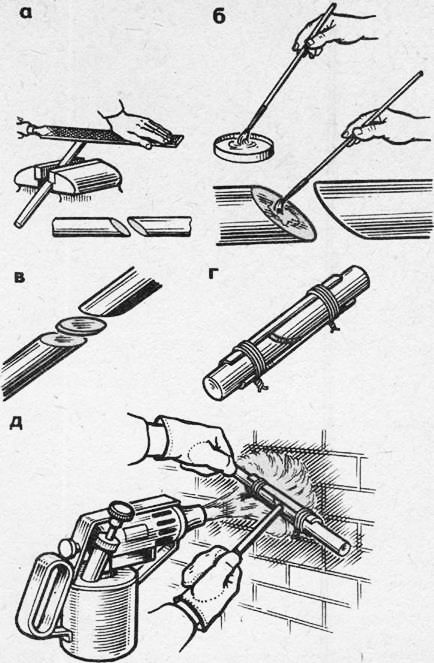
Rice. 2. Hard soldering: a - adjusting the surfaces of parts, b - lubricating the surfaces of parts with flux, c - inserting a copper plate, d - fixing the parts to be connected with a guide gasket, e - heating the parts
There are several methods for brazing. These methods can be classified according to the way the metal is heated during the soldering process. Typically, brazing solders are divided into copper, copper-zinc, copper-nickel and silver. A separate group consists of aluminum solders. The most important brazing alloys are standardized.
PMTSZ solder b is not used in mechanical engineering due to its low strength and fragility. Solders PMTs48 and PMTs54 are rarely used due to insufficient ductility and low vibration resistance of the joints they solder. The most widely used solders are JI62 and JIOK 62-06-04, which give strong solder connections. The tensile strength of JI62 solder is 30 kg/mm2 with an elongation of 35%.
The basis of most fluxes for hard soldering is Na2B407 borax, which crystallizes with ten parts of water into large transparent colorless crystals of Na2B407 YN20. Crystalline borax begins to melt at 75 °C; as
Increasing heating, it gradually loses water, strongly swelling and splashing, and turns into anhydrous salt - melted or burnt borax, melting at a temperature of 783 ° C. Borax in its molten state can be heated to high temperatures without noticeable evaporation; it is very fluid and energetically dissolves the oxides of many metals, especially copper oxides.
For soldering stainless steel, a mixture of equal parts drills and boric acid, mixed with a saturated aqueous solution of zinc chloride to a paste. When soldering gray malleable cast iron to burn off graphite and increase net metal surface, wetted with solder, strong oxidizing agents (potassium chlorate, manganese peroxide, iron oxide, etc.) are often introduced into fluxes.
Fluxes can be in powder or paste form. Fluxes are also used in the form of liquid solutions, for example a solution of borax in hot water. Sometimes it is advisable to use solder rods coated with flux. The fluxing effect can be exerted by the components of the solder itself. For example, phosphorus, oxidized into phosphoric anhydride, is a good flux for copper and copper alloys, reducing oxides and converting them into fusible phosphoric acid compounds. Therefore, phosphorous copper iripoi do not require fluxes for soldering copper alloys, which is very convenient in practice.
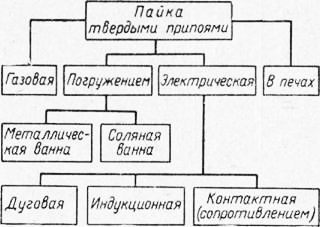
Rice. 1. Classification of brazing methods
Powdered fluxes can be sprinkled thin layer on the edges, and preheating of the edges is often used so that the flux particles melt, sticking to the metal, and are not blown away by the burner flame during soldering. The end of a solder rod, heated above the melting point of the flux, can also be dipped into powdered flux, which firmly adheres to the rod. Pastes and liquid solutions are applied with a brush or solder is dipped into them. You can make a paste of flux and powdered solder and apply it to the edge before soldering.
For soldering they have important preparatory work, often determining the quality of the connection. Three main forms of solder joints are widely used: lap, butt and miter joints (Fig. 239). The most common is the lap joint, which is easy to perform and very durable. By increasing the overlap of the lap joint, it is possible to increase its strength and, in most cases, achieve equal strength with the base metal. The butt joint has the best appearance and with good solders and correct execution can often provide sufficient strength (tensile strength can reach 40-45 kg/mm2). Butt joints are used in cases where doubling the thickness of the metal is undesirable. The miter joint, which requires advanced edge preparation, combines the advantages of butt and lap joints and provides a good appearance and no protruding edges. A miter connection makes it possible to achieve equal strength with the entire section by increasing the working area of the connection.
Of significant importance is the size of the gap between the edges being joined, which should be small both to improve the absorption of liquid solder by capillary forces and to increase the strength of the connection. For silver solders, a gap of 0.05-0.15 mm is recommended; For soldering with copper in shielding gas, gaps of 0.1-0.2 mm are recommended. Strict requirements regarding the gap size force the production of fairly clean machining surfaces, since rough processing, such as filing or sandblasting, can cause excessive consumption of solder in the joint and a sharp drop in its strength.
For good solder wetting, the surface to be soldered must be immaculately clean. You can degrease with hot alkali, trichlorethylene or carbon tetrachloride. Oxides are removed by etching in acids, followed by thorough washing and drying.
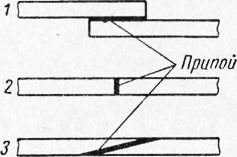
Rice. 2. Forms of solder joints: 1 - overlap; 2 - butt; z - “in the mouth”
Mechanical cleaning is carried out by wiping with a rag, fine sandpaper, grinding with fine-grained grinding wheels, brushes, etc. During assembly, flux is often first applied to the edges and solder is placed between the edges; in this case, solder is used in the form of foil or fine powder, or solder in the form of a wire or tape placed near the soldering site.
Before soldering, the assembled parts must be sufficiently firmly fastened with wire ties, pins, spot welding, etc., in order to eliminate the possibility of parts moving during heating and during the soldering process. The surface of products that should not be tinned is coated before soldering with a paste of chalk, clay, graphite or mixtures thereof, or moistened with a solution of chromic acid and similar substances that eliminate the adhesion of solder to the surface of the product.
Brazing alloys consist mainly of copper, silver and zinc. They are used for soldering both ferrous and non-ferrous metals and their alloys.
There are many different chemical composition hard solders. The group of hard solders includes copper-zinc and silver.
Copper-zinc solders. Copper-zinc solders contain copper and zinc. Depending on the copper and zinc content they have different properties. The more copper the solder contains, the higher its melting point, and, conversely, the more zinc and less copper it contains, the lower the melting point of the solder. In addition, copper-zinc solders contain lead and iron in amounts up to 1.5%. The addition of lead makes solders lighter.
According to GOST 1534-42, copper-zinc solders are used in three grades: PMC-36, PMC-48 and PMC-54. In the brand, the letter P stands for the word “solder,” MC stands for copper-zinc, and the number stands for the percentage of copper. Copper-zinc solders are supplied in grain form. Solder grains are divided into two classes by size: class A - grains ranging in size from 0.2 to 3 mm, class B - grains ranging in size from 3 to 5 mm.
Copper-zinc solder PMC-36 is used for soldering brass containing 60-68% copper; solder PMTs-48 - for soldering copper alloys containing copper over 68%; PMC-54 solder - for soldering bronze, copper, tombac and steel.
Copper-zinc solders are unsuitable for soldering products subject to high internal pressure. This is explained by the fact that copper-zinc alloys lose zinc before solidification, so small pores form in the weld. In these cases, pure copper solder is used. Pure copper is the best solder for strong and tight connections of steel products. It is used in the form of wire, powder or tape and melts at a temperature of 1083 ° C. The disadvantage of copper as a solder is that soldering is carried out at high temperatures, which increases the cost of soldering.
Silver solders. Silver solders are basically alloys of silver with zinc and copper. Their melting point increases with increasing percentage of silver. They form a very strong connection metal products. Silver solders are used to increase corrosion resistance, or in cases where it is necessary to preserve light color products.
According to GOST 8190-56, silver solders are produced in the following grades: PSr72; PSr71; PSr70; PSr65; PSr62; PSr50; PSr50Kd; PSr45; PSr44; PSr40; PSr37.5; PSr25; Sr25F; PSr15; PSr12M; PSr10; PSr3; PSr3Kd; PSr2.5; PSr2; PS1.5. Brands of silver solders are deciphered as follows: the letter P stands for the word “solder”, the letters Sr - silver, Kd - cadmium, M - copper, F - phosphorus, number - percentage of silver.
Silver solders melt at temperatures from 270 to 850° C. These solders are manufactured in the form of strips (with the exception of PSr44 solder, produced in the form of flat ingots) and wire (with the exception of PSr12M, PSr10 solders).
Page 1
Refractory solders (Table 34) are widely used for so-called hard soldering in the production of various electrical equipment, automatic devices, apparatus and devices.
Refractory solders melt at 550 - 950 C. All connections when installing air separation units are usually made by hard soldering.
Refractory solders provide high strength connections.
High-strength refractory solders are also called hard solders, and low-melting solders are called soft solders.
The simplest refractory solder is pure copper.
The simplest refractory solder is pure copper. Connections soldered with copper have high strength and ductility.
Pure copper is a strong and ductile refractory solder, but its melting point is very high, so it is used only for soldering steel products.
There are low-melting and high-melting solders.
There are low-melting and high-melting solders. TO low melting point solders with a melting point of up to 300 C include tin-lead alloys. To lower the melting point, bismuth and cadmium are introduced into these alloys, and antimony is added to increase strength. Refractory solders contain copper, zinc, silver and have a melting point above 500 C.
Among refractory solders, silver and brass solders are successfully used for soldering cast iron; to increase the strength of the connection, brass is often added a large number of(1 0 - 1 5%) silicon, tin, nickel, manganese or iron. Copper for soldering cast iron should be used carefully due to its high melting point, and solders containing phosphorus should not be used at all due to the formation of brittle iron-phosphorus compounds. Cast iron parts operating at high temperatures are soldered with copper-nickel alloys or nickel silver.
Among refractory solders, silver and brass solders are successfully used for soldering cast iron; To increase the strength of the connection, small amounts (10 - 15%) of silicon, tin, nickel, manganese or iron are often added to brasses.
Of the refractory solders for soldering cast iron, silver solders and brass are successfully used; to increase the strength of the connection, a small amount (10 - 15%) of silicon, tin, nickel, manganese or iron is often added to brass.
Refractory solders, also called hard solders, include solders with a melting point above 400 - 500 C. Table. 76 and 77 show two groups of such solders from among those accepted at instrument-making plants: I) solders on copper base; 2) silver solders.
Refractory solders, also called hard solders, include solders with a melting point above 400 - 500 C. Table. 53 and 54 show two groups of such solders from those accepted at instrument-making plants: 1) copper-based solders; 2) silver solders.
Soft solders
Solders
Solders are used when soldering metals. Unlike welding, when the edges of the products being joined are melted, during soldering the metals are heated only to the melting temperature of the solder, and the metals being soldered do not melt, but dissolve in the solder. The soldering strength depends on the depth of mutual penetration of the contacting materials. To ensure diffusion processes, the solder must well wet the surface of the metals being soldered and flow well into the gap formed by the edge of the products.
Solders are divided into soft and hard, distinguished by melting temperatures.
Soft solders include alloys of tin and lead with a melting point of up to 350°C. Soft solders have good wettability and fluidity. Of these, the most common POS-90 (89…90% Sn; 0.10…0.15% Sb; ost. Pb ), with a melting point of 222°C. Soft solders are used for soldering household utensils, canned food containers and medical equipment. Solder POS-40 (39…40% Sn; 1.5...2% Sb; ost. Pb ) with a melting point of 235°C is used for soldering copper, iron and brass products, as well as for electrical equipment. Solder POS-30 (29…30% Sn; 1.5...2% Sb; ost. Pb ; T pl = 256°C) are used for soldering brass, copper, zinc, galvanized sheet, tinplate, radio equipment. Solder POS-18 (17…18% Sn; 2…2.5% Sb; ost. Pb; T pl = 277 °C) are used for soldering consumer goods, tinning iron, soldering lead, brass, and copper.
As the lead content in solders increases, the solder strength of most materials decreases. For low-temperature soldering, tin-zinc solders are also used, which are marked as POC . Solder POTs-90 (90% Sn, 10% Zn ) has the lowest melting point, which is 200 0 C. Solders of this series ( POTs-60, POTs-70, POTs-90 ) are used for soldering aluminum and its alloys.
Before soldering, the surfaces to be joined are cleaned with sandpaper, then treated with flux, which is often used as zinc chloride. For soldering zinc and zinc alloys, use a 10% solution instead of flux. of hydrochloric acid, and when soldering copper - rosin. When soldering with soft solders, preheating is necessary to facilitate diffusion and produce strong joints.
Hard solders include copper-zinc, copper-phosphorus and copper-silver-zinc. Hard solders are used to solder steel, cast iron, copper, and bronze. One of them is PMC-36 (36…30% Cu; ost. Zn; T pl = 833 °C ). Copper-zinc solders also include PMC-48 And PMC-54 . The first of them contains 46…50% Cu , in the second - 52…56% Cu . Their melting points are 850 and 870 °C, respectively.
Copper phosphorus solders, for example PMF-7 (7% P ; ost. Cu ), allow you to solder copper without using flux, which simplifies and speeds up the process. Silver solders, the main ones of which PSr-12 (36% C; 52% Zn; 12% Ag; T pl = 785 °C), PSr-25 (40% Cu; 35% Zn; 25% Ag; T pl = 765 °C ), PSr-45 (30% Cu; 25% Zn; 45% Ag; T pl = 720 °C ) are used in the form of rods, strips, grains.
For soldering steel parts, it is recommended to use solders with a lower zinc content; for soldering copper alloys, on the contrary, with a higher zinc content. Silver solders not only have good fluidity and corrosion resistance, but also provide strong connections that can withstand significant shock and vibration loads.
When brazing, the surfaces to be soldered must also be thoroughly cleaned. Borax, boric acid and their mixtures are used as flux. When soldering aluminum and its alloys, a 30% alcohol solution of a mixture consisting of 90% ZnСl 2; 2% NaCl And 8% AlCl 3 .
In the manufacture of steel products, copper soldering is often used in special electric ovens with a protective atmosphere. In this case, the parts of the soldered assemblies are assembled together and placed in place of the seams. copper wire or tape. Copper heated to 1150...1200 °C flows into the seams. Sometimes soldering is carried out in oil or gas furnaces. In this case, it is advisable to use fluxes to remove soot. When soldering is carried out in salt bath furnaces, molten salts protect the metal from oxidation, and therefore soldering can be carried out without filling the seams with flux.
The division of soldering into low-temperature and high-temperature is, to some extent, conditional. By its physical nature, hard soldering is no different from soft soldering. Like the latter, it is the process of forming a permanent connection of two metals with the help of a third (called solder), the melting point of which is lower than the melting temperature of the metals being connected.
And yet, despite the fact that low-temperature and high-temperature soldering are phenomena of the same essence, their technology, the materials and equipment used, and the characteristics of the resulting connection are significantly different. Which, in fact, was the basis for separating these methods. The boundary temperature separating them is taken to be 450°C.
Differences between high-temperature soldering and low-temperature soldering
What distinguishes high-temperature soldering from low-temperature soldering, except for the melting point of the solders? First of all, a significantly higher strength of the soldered joint, due to the greater strength of hard solders compared to soft ones.
An important difference between high-temperature soldering and low-temperature soldering is the increased thermal stability of the connection. Since the melting point of hard solders is much higher than the melting point of soft solders, a connection made by high-temperature soldering is able to operate at higher temperatures while maintaining all its properties. In many cases, when choosing a soldering method, this feature is decisive.
But there are also ways in which hard soldering is inferior to soft soldering. Relatively high temperatures can cause structural changes in some metals. This, in particular, is observed in cast iron, in which, during soldering, hardening structures can appear, leading to increased fragility of the metal in the weld zone.
The high melting point of hard solders places its own demands on heating sources. They must ensure the melting of solders, the melting point of which sometimes reaches 1000°C. This excludes the use of convenient soldering irons for high-temperature soldering, which are the main tool for soft soldering.
Summarizing the above, we can summarize the comparison of high-temperature and low-temperature soldering. The advantages of the first include high strength and thermal stability of the connection, the disadvantages are complexity technological process, due to the need to heat the soldered parts to relatively high temperatures.
Applications of brazing
The scope of application of brazing is determined by its intermediate position between low-temperature soldering and welding. Wherever it is required to obtain a more durable connection than can be done using soft solders, which can also work at high temperatures, and at the same time preserve the structure of the metals being connected, preventing their softening and deformation (as is the case with welding), high-temperature soldering is used.Brazing is the main method in the manufacture of metal-cutting tools with carbide inserts. Soldering the latter provides sufficient strength of the connection and does not have a negative impact on the hardness and geometry of the cutting inserts.

Production of all kinds of vessels from non-ferrous metals and stainless steels, connection of steel and copper pipelines operating under high pressure or elevated temperature in various systems- refrigeration, heat exchange, etc. - also cannot do without hard soldering.
High-temperature soldering is widely used in car repairs - radiators, engine and transmission piping systems, bodies, various parts - wherever it is impossible or undesirable to use welding.
It is advisable to use high-temperature soldering to connect thin-walled parts that operate under significant loads and elastic deformations.
For the repair of copper and brass household products that are exposed to high temperatures during operation, high-temperature soldering is a repair method that has no alternative. Such, for example, as an ancient samovar, heated with wood. In this case, soft solders cannot be used due to their inability to withstand high temperature heating
Heating sources for high-temperature soldering
Any equipment that allows heating the soldered parts slightly above the melting point of the solders used can be used as heating sources for high-temperature soldering. This temperature can range from 450-1200°C. When using refractory materials such as brass or commercially pure copper, heating in excess of 1000°C is required; when using medium-melting solders, a heating temperature of 700-800°C is required.The main sources of heat during high-temperature soldering are gas-burners various types, inductors and furnaces. Electrical resistance heating is also used. In everyday life, hard solders are most often soldered using torches.
Solders
The main merit in the formation of strong and heat-resistant joints during high-temperature soldering belongs to copper. Not only is it included in almost all hard solders, but in most of them it performs main role, being the basis of solders.Sometimes commercially pure copper is also used as solder. However, much more often they use soldering with copper solders, which are compounds of copper with other metals - zinc, silver, silicon, tin, etc. Each of these elements contributes to the technological properties of solders. Almost all of them reduce the melting point (for pure copper it is 1083°C).
For high-temperature soldering, copper-zinc, copper-phosphorus, silver and brass solders are used.

Copper-zinc solders. There is a large number of copper-zinc solders (PMC-35, PMC-39, PMC-50, PMC-54, PMC-57, etc.). The numbers indicate the percentage of copper. They are used for soldering bronze, copper, and steel. The disadvantage of pure copper-zinc materials is bad job under conditions of shock, vibration and bending loads. To remove or reduce this disadvantage, alloying them with other metals is used (for example, brass can be considered as alloyed copper-zinc solders). Alloyed copper-zinc solders are used, in particular, when soldering carbide cutters.
Copper-phosphorus solders. Copper-phosphorus solders (PMF-7, PMF-9, PMFOTsr-6-4-0.03) are an alloy of copper and phosphorus. The number following the letters indicates the percentage of phosphorus. Solder PMFOTsr-6-4-0.03, in addition to copper and phosphorus, contains tin and zirconium.
Copper-phosphorus solders are medium-melting (700-850°C), have high fluidity and good corrosion resistance to aggressive environments. Used for soldering copper and its alloys (bronze, brass, cupronickel). They can also be used as a substitute for silver solders when repairing jewelry.
Soldering of steel and cast iron with copper solders containing phosphorus is not used due to the increased fragility of the joint and its inability to withstand shock, vibration and bending loads. This is caused by the formation of a film of phosphites along the seam boundary.
A distinctive feature of copper-phosphorus solders is that they are self-fluxing. When soldering copper products with them, the use of flux is not necessary.
Brass. Brasses, which are an alloy of copper and zinc, are widely used as solders. Brasses L62 and LOK-62-06-04 provide strong solder joints. LOK-62-06-04 differs from L62 in the presence of tin and silicon, which provide higher technological properties of the solder. Tin increases fluidity and reduces the melting point, and silicon compounds protect zinc from oxidation and evaporation. Brasses are used for soldering copper, steel, and cast iron.
Silver solders. Silver is an excellent material for soldering. Silver solders, which are basically an alloy of silver with copper and zinc, rank first in spreading, wettability, strength and anti-corrosion properties. If they weren't so expensive, we could eliminate all other solders and use only silver ones. Fortunately, they are versatile and can solder almost any metal.
Silver-based solders are designated by the letters PSr (PSr-15, PSr-25, PSr-45, PSr-65, PSr-70). The Psr-15 and Psr-25 grades are used for soldering not very critical parts. If you want to get a particularly high-quality connection, use PSR-45 solder, which has 45% silver, 30% copper and 25% zinc. PSR-45 has excellent qualities- viscosity, malleability, fluidity, resistance to corrosion, ability to withstand vibration and shock. PSR-65 solder is not inferior to PSR-45, but is too expensive.
Silver solders can be used to solder almost any metal - copper and its alloys, silver, steel, etc. However, due to their high cost, soldering with silver solders is used only where it is economically feasible, in particular, for joining stainless steels that are difficult to solder and require solders that have good wettability and avoid corrosion that may occur at the solder joint.
Fluxes
The main component of fluxes for hard soldering are boron compounds - borax (Na 2 B 4 O 7), boric acid (H 3 BO 3), boric anhydride (B 2 O 3). To enhance the activity of boron fluxes, for example when soldering stainless and heat-resistant steels, fluorine compounds are added to them - calcium fluoride, potassium fluoride. Special fluxes are used, regulated by GOST 23178-78 - under the brands PV200, PV201, PV209, PV209X, PV284X. The first two include boric acid, borax and calcium fluoride. They are used for soldering stainless and structural steels and heat-resistant alloys. Flux PV209 consists of potassium fluoride, boric anhydride, potassium tetrafluoroborate. Fluxes PV209X, PV284X consist of boric acid, potassium hydroxide, and hydrofluoric acid. Fluxes PV209, PV209X, PV284X can be used for soldering copper and its alloys, stainless and structural steels.Soldering of copper and its alloys can be done using pure borax, which is a universal flux for high-temperature soldering.

Various forms of flux are used - liquids, powder, pieces (borax crystals, for example). To facilitate their dosing (an excess of flux is just as undesirable as a deficiency), they are combined with solder. This is done different ways- adding in powder form to loose forms solders, coating solder rods or placing solder tubes inside, jointly pressing tablet forms.
High temperature soldering technology
In the given example, parts are selected as soldered parts wrench. As solder, it is a material that is a rod coated with flux. A highly active flux suitable for stainless steels is also required. The heating tool is a gas burner.
Soldering is performed in this sequence. The butt parts of the parts are cleaned mechanically. The operation is necessary to remove the persistent oxide film that covers stainless steels.

The parts are clamped in a vice in the required position.

The soldering area is coated with flux.

The burner is ignited and the required combustion mode is set. The flame should be reducing, with a slight lack of oxygen (but not soot and yellow fire). A flame supersaturated with oxygen oxidizes the metal surface.
The soldered area is heated until the color of the part begins to change (when touched, the flux on the rod should begin to melt). You need to warm up the entire connection, moving the flame in different directions.

The joint is fluxed with flux from the rod - by friction of the latter along the joint. If a non-fluxed rod is used, after the tip has been heated, it must be dipped in flux to coat it.

The heating of the soldering zone is brought to a cherry color. Typically, brazing is done in a range of colors from dark cherry to light cherry.

Heating parts to a higher temperature High-temperature soldered wrench

When using the content of this site, you need to put active links to this site, visible to users and search robots.




