How to connect aluminum and copper wires correctly? Ways to connect aluminum and copper wires, how to connect the wires correctly, expert advice.
When repairing electrical wiring in old houses, you may encounter a situation where you have to change large sections of wiring. However, in most cases, the old wiring is made of aluminum, and you only have copper wire for replacement. In general, to connect conductors from such different materials strictly prohibited, but it happens that there is simply no other way out. Consider how to nevertheless connect the aluminum and copper wire so that there is no short circuit or fire.
To do this, strain your memory and remember school course chemistry and physics.
First, let's remember what is galvanic cell. Simply put, galvanic element is simple battery, which generates electricity. The principle of its appearance is based on the interaction of two metals in the electrolyte. So, twisting between copper and aluminum wire will be the same battery.
Galvanic currents quickly destroy the material. True, in dry air their appearance is excluded. And if you make a twist to the outlet, then it will not fall apart in a few hours. However, subsequently the troubles of such wiring are provided.
Over time, the materials from which the wires are made are destroyed, along with this, constantly resistance increases. If a powerful current consumer is connected to the outlet, the twist will begin to heat up. At regular use such an outlet increases the risk of fire.
Therefore, it is strictly forbidden to connect an aluminum conductor with a copper conductor. However, there are emergency situations when it is simply necessary to make such a connection.
Consider several ways how to connect aluminum and copper wire. These methods will help you successfully cope with a difficult task.
Twisting
 Is most in a simple way
mount wires. It does not require special knowledge and qualifications. However, it is not the most reliable connection method. Because of temperature fluctuations metal expands. As a result, a gap is formed between the conductors, increasing the resistance. After some time, the contact is oxidized and destroyed.
Is most in a simple way
mount wires. It does not require special knowledge and qualifications. However, it is not the most reliable connection method. Because of temperature fluctuations metal expands. As a result, a gap is formed between the conductors, increasing the resistance. After some time, the contact is oxidized and destroyed.
Of course, this will not happen within a year, but if the connection must function for a long time, then it is worth considering other methods of fastening.
The very principle of fastening by twisting is that both conductors wrapped around each other. For a better connection copper cable tinned with solder. Stranded copper wire will have to be tinned without fail.
Threaded connection
To connect copper and aluminum in this way, you need a pair of plain washers, one spring washer, screw and nut. This method is very reliable - contact between the conductors will be ensured for many years. For this fastening, neither the cross section of the wire, nor its type - stranded or solid.
The insulation is removed from the end of the wire. A spring washer is put on the screw, then a regular washer is put on, then a ring of aluminum wire. It is supported by a simple washer. After that, a copper conductor is put on, and then a nut is screwed onto the screw. It tightly compresses the entire connection.
Stranded cable must be soldered before connection.
Connection via terminal block
This modern method wiring installation. Although it loses a little in reliability to the threaded connection method , the method has its advantages:
- connection can be made very quickly;
- when connecting, you can get by with a small supply of wire.
Let us explain the latter, it happens that a small piece of cable sticks out of a wall or ceiling. It is impossible to make a twist - there are very few wires. And the twist made on the ceiling will not last long, after some time the wires will simply break off. And the terminal block will hold both conductors with screws for a long time. Then the block completely eliminates the contact of the two stripped conductors.
Installation is carried out as follows: the end of the wire stripped of insulation (about 5 mm.) Is inserted into the terminal hole of the block, after which locking screw is tightened.
The terminal block must not be hidden in plaster or in a wall without a junction box.
Flat spring clamp and terminal block
This method appeared not so long ago. There are two types of such a connection: disposable and reusable. For the last connection in the terminal block there is a special lever. Thanks to him, the wire can be inserted and removed several times. Terminal blocks of this type can successfully connect copper and aluminum stranded wires of various types.
Widely used for installing chandeliers and connecting wires in junction boxes. Some force is required to insert the wire into the hole in the terminal block. It will take even more effort to pull the conductor out. For practical application it is better to use reusable models. In the event of an error, the connection can be quickly redone.
This installation is very easy to do. First with the cable the insulation is removed(about 10 mm.). Then, on the reusable terminal block, you need to lift the lever, insert the wire, and then return the lever to its original position. Everything is simple!
Rivet
In terms of reliability, it is not inferior to a threaded connection and has its own Advantages and disadvantages:
- such a connection is established very quickly;
- it is very durable, reliable and affordable;
- however, unlike threaded fasteners, this connection is disposable.
Installation is done using special tool- a riveter. An aluminum wire is put on the rivet, then a spring nut, then a copper wire and a flat washer. Then the riveter comes into play and the connection is ready.
It is worth mentioning that the connection site must be isolated.
Soldering
Is it possible to solder conductors made of various materials? Quite possible if meet certain conditions.
There will be no problems with soldering copper, unlike aluminum. An amalgam is formed on the surface of this metal, which exhibits amazing chemical resistance. That is, the solder cannot stick to it. This phenomenon often comes as a surprise to novice electricians.
To solder two different conductors, you should stock up on a solution of copper sulfate, a Krona battery and a piece copper wire. On the aluminum wire, the future place of soldering is carefully cleaned. Then this place is dripped copper sulphate solution.
The copper wire is connected to the positive pole of the Krona battery and lowered into blue vitriol. An aluminum conductor is connected to the negative pole of the battery. After a while, a layer of copper will settle on aluminum, on which you can solder the desired wire without any problems.
Conclusion
Once again, it is worth noting that any wire connection must be insulated.
Connections can be placed in special junction boxes .
If the connection is to be made with my own hands, then you should not resort to the soldering method. It requires certain experience and qualifications. It is better to use another of the above methods for connecting aluminum and copper conductors.
The most accessible and common methods were discussed in the article. However, in the absence of experience in carrying out such work, it is better to turn to professionals.
What is in electrical engineering Do not directly connect copper and aluminum conductors, is not a secret even for many ordinary people who have nothing to do with electrics. From the side of the same inhabitants, the question is often asked to professional electricians: “Why?”.
Why chicks of any age are able to drive anyone into a dead end. Here and here similar case. A typical professional answer: “Why, why ... Because it will burn. Especially if the current is large. But this doesn't always help. Since this is often followed by another question: “Why will it burn? Why doesn't copper and steel burn, aluminum and steel don't burn, and aluminum and copper don't burn?
There are different answers to the last question. Here are some of them:
1) Aluminum and copper have different coefficients of thermal expansion. When a current passes through them, they expand in different ways, when the current stops, they cool down in different ways. As a result, a series of expansion-narrowing changes the geometry of the conductors, and the contact becomes loose. And then heating occurs in the place, it worsens even more, an electric arc appears, which completes the whole thing.
2) Aluminum forms an oxide non-conductive film on its surface, which from the very beginning worsens the contact, and then the process goes along the same increasing path: heating, further deterioration of the contact, arc and destruction.
3) Aluminum and copper form a "galvanic couple", which simply cannot help but overheat at the point of contact. And again heating, arc and so on.
Where is the truth, after all? What happens there, at the junction of copper and aluminum?
The first of the given answers all the same is inconsistent. Here are the tabular data linear coefficient thermal expansion for metals used for electrical installation: copper - 16.6 * 10-6m / (m * gr. Celsius); aluminum - 22.2 * 10-6m / (m * gr. Celsius); steel - 10.8 * 10-6m / (m * gr. Celsius).
Obviously, if it were a matter of expansion coefficients, then the most unreliable contact would be between a steel and aluminum conductor, because their expansion coefficients differ by a factor of two.
But even without tabular data, it is clear that differences in linear thermal expansion are relatively easily compensated for by using reliable clamps that create a constant pressure on the contact. To expand to metals compressed, for example, with the help of a well-tightened bolted connection, remains only to the side, and temperature changes are not able to seriously weaken the contact.
The option with an oxide film is also not entirely correct. After all, the same oxide film allows you to connect aluminum conductors with steel and with other aluminum conductors. Yes, of course, the use of a special lubricant against oxides is recommended, yes, a systematic revision of compounds involving aluminum is recommended. But all this is allowed and works for years.
But the version with a galvanic pair really has a right to exist. But here it still does not do without oxides. After all, the copper conductor is also quickly covered with oxide, with the only difference being that copper oxide more or less conducts current.
During electrolysis, ions transfer charges and move themselves. But, in addition, ions are, after all, particles of metal conductors. When they move, the metal is destroyed, shells and voids are formed. This is especially true for aluminum. Well, where there are voids and shells, it is no longer possible to have a reliable electrical contact. A bad contact starts to warm up, it gets even worse, and so on up to a fire.
Note that the more humid the ambient air, the more intensively all of the above processes proceed. And uneven thermal expansion and a non-conductive layer of aluminum oxide are only aggravating factors, nothing more.
The connection of wires from dissimilar metals (a particular and most common case is copper with aluminum) is most often necessary in cases where home wiring is made of copper conductor, and the entry to the house is made of aluminum.
It happens the other way around. The main thing here is the contact of dissimilar metals. Direct combination of copper and aluminum cannot be performed.
The reasons lie in the electrochemical properties of metals. Most metals, when combined with each other in the presence of an electrolyte (water is a universal electrolyte), form something like a conventional battery. For different metals, the potential difference during their contact is different.
For copper and aluminum, this difference is 0.65 mV. It is established by the standard that the maximum allowable difference should be no more than 0.6 mV.
In the presence of a higher potential, the material of the conductors begins to break down, covered with oxide films. Contact will soon lose reliability.
For example, the electrochemical potential difference of some other metal pairs is:
- copper - lead-tin solder 25 mV;
- aluminum - lead-tin solder 40 mV;
- copper - steel 40 mV;
- aluminum - steel 20 mV;
- copper - zinc 85 mV;
Wire twisting
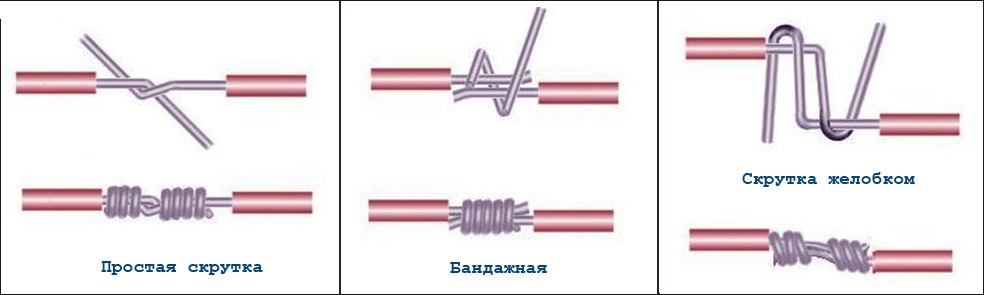
The simplest, but least reliable way to connect conductors. As mentioned above, copper and aluminum wire cannot be twisted directly. The only one possible variant contact of such materials - tinning one of the conductors with lead-tin solder.
It is very difficult to irradiate aluminum at home, but there will be no problems with copper. Powerful enough, a piece of solder and a little rosin or other flux for soldering copper and copper alloys. Tinned copper and pure aluminum conductors are tightly twisted together with pliers or pliers so that the cores wrap tightly and evenly around each other.
It is unacceptable for one conductor to be straight and the other to wrap around it. The number of turns should be at least 3-5. The thicker the conductors, the smaller the number of turns can be made. For reliability, the place of twisting can be wrapped around with a bandage of thinner tinned copper wire and additionally soldered. The place of twisting must be carefully insulated.
Threaded connection

The most reliable connection of wires is threaded (bolted). The conductors are pressed against each other by means of a bolt and nut. To make such a connection at the ends of the wires to be connected, it is necessary to make rings with inside diameter equal to the diameter of the bolt.
As well as for twisting, the copper core must be tinned. Must be serviced stranded wire(even if wires of the same metal are connected).
The resulting connection looks like a sandwich:
- bolt head;
- washer (outer diameter not less than the diameter of the ring on the wire);
- one of the connected wires;
- second wire;
- washer similar to the first;
- screw;
The copper core may not be tinned, but in this case a steel washer must be laid between the conductors.
A significant drawback of this method is its large dimensions and, as a result, difficulties with insulation.
Terminal blocks
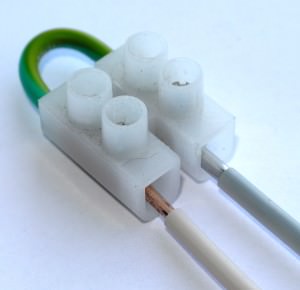 The most technologically advanced way to connect wires is to use special terminal blocks.
The most technologically advanced way to connect wires is to use special terminal blocks.

And finally, a few tips that you should take into account in order to protect yourself in the future and not redo the work:
- For stripping conductors do not use side cutters, pliers or other tools with a similar principle of operation. In order to cut the insulation without affecting the body of the wire, considerable experience is required and in most cases the integrity of the wire will still be compromised. Aluminum is a soft metal, but it does not tolerate kinks very well, especially if the integrity of the surface is compromised. It is possible that the wire breaks already during the installation process. And much worse if it happens a little later. It is necessary to remove the insulation sharp knife, moving it along the conductor, like stripping a pencil. Even if the edge of the knife removes some layer of metal, a scratch along the wire is not terrible.
- For tinning copper conductors in no case should acidic fluxes (zinc chloride, pickled hydrochloric acid and so on). Even thorough cleaning connection will not save it from destruction for some time.
- stranded conductors before installation, it is necessary to irradiate to obtain a monolithic conductor. The only exceptions are spring clips and terminal blocks with pressure plates.
- Washers, nuts and bolts for detachable or permanent connections should not be made of galvanized metal. The potential difference copper - zinc is 0.85 mV, which is much greater than the difference in the direct connection of copper and aluminum.
- For the same reason, you should not purchase overly cheap terminal blocks. unknown manufacturer. Practice shows that metal elements these pads are often zinc coated.
- Can't take advice protect the direct connection of copper and aluminum conductors with various water-repellent coatings (grease, paraffin). It is difficult to remove machine oil only from the skin. sun, air, negative temperatures destroy protective covering much faster than we would like. In addition, some lubricants (especially fatty grease) initially contain up to 3% water in their composition.
1. If a permanent magnet is pushed into the coil and an electric current appears in it, then this phenomenon is called:
A. Electrostatic induction B. Magnetic induction
C. Inductance D. Electromagnetic induction
D. Self-induction
2. Inductance in the SI system has the dimension:
A. C B. Tl C. Gn G. Wb D. F
3. Flux of magnetic induction through a surface area S is determined by the formula:
A. BS B. BScos IN. G. BStg D.
4. The rate of change of the magnetic flux through the circuit determines:
A. Circuit inductance B. Magnetic induction
C. EMF of induction D. EMF of self-induction
D. Electrical resistance contour
5. The magnetic flux through a circuit with an area of 10 cm2 is 40 mWb. The angle between the induction vectors and the normal is 60 . Induction module magnetic field equals:
A. 2∙10-5 T B. 8∙105 T C. 80 T D. 8 T E. 20 T
6. Driving permanent magnet the needle of the galvanometer deviates into the coil. If the speed of the magnet is increased, then the angle of deviation of the arrow:
A. Decrease B. Increase C. Reverse
D. Will not change E. Will become zero
7. When the current in the coil decreases by 2 times, the energy of its magnetic field:
A. It will decrease by 2 times B. It will increase by 2 times
C. Decrease by 4 times D. Increase by 4 times
D. Will not change
8. August 29, 1831 The phenomenon of electromagnetic induction was discovered:
A. Oersted H. B. Lenz E. W. Ampere A.
G. Faraday M. D. Maxwell D.
9. If, at a current strength of 3 A, a magnetic flux of 600 mWb occurs in the frame, then the inductance of the frame is:
A. 200 Gn B. 5 Gn C. 0.2 Gn D. 5∙10-3 Gn E. 1.8 Gn
10. The self-induction emf that occurs in a coil with an inductance of 0.2 H with a uniform change in current from 5 A to 1 A in 2 s is equal to:
A. 1.6 C B. 0.4 C C. 10 C D. 1 E. D. 2.5 C
11. In a coil made of aluminum wire (=0.028 Ohm∙mm2/m) with a length of 10 cm and a cross-sectional area of 1.4 mm2, the rate of change of the magnetic flux is 10 mWb/s. The strength of the induction current is:
A. 50 A B. 2.5 A C. 10 A D. 5 A E. 0.2 A
12. A straight conductor with a length of 1.4 mi with a resistance of 2 ohms, located in a uniform magnetic field with an induction of 0.25 T, is subjected to a force of 2.1 N. The voltage at the ends of the conductor is 24 V, the angle between the conductor and the direction of the induction vector is:
A. 0 B. 30 C. 60 D. 45 E. 90
13. In a coil with 1000 turns, with a uniform disappearance of the magnetic field for 0.1 s, an EMF equal to 10 V is induced. The flux penetrating each turn of the coil is equal to:
A. 10 Wb B. 1 Wb C. 0.1 Wb D. 10-2 Wb E. 10-3 Wb
14. A coil in the form of a solenoid with a cross section of 10 cm2 is placed in a uniform magnetic field, the induction of which changes with time, as shown in the graph. The magnetic induction vector is parallel to the coil axis. How many turns does the coil have if at the time t=3 with an induction emf equal to 0.01 V?
A. 20 B. 50 C. 100 D. 200 E. 150
15. Coil diameter d, which has N turns, is in a magnetic field directed parallel to the axis of the coil. What is the average value of the induction EMF in the coil if the magnetic field induction over time t increased from 0 to B?
A B C D E.
16. If, with a uniform decrease in current strength by 0.2 A in 0.04 s, an EMF of self-induction equal to 10 V occurs in the coil, then the inductance of the coil is ...
Palamedea / June 24, 2014, 11:48:29 PM
1, A current of 1 A flows through the conductor during the year. Find the mass of electrons that have passed through the cross section during this period of timeconductor. The ratio of an electron's charge to its mass e/te\u003d 1.76 * 10 ^ 11 C / kg.
2, In a conductor with a cross-sectional area of 1 mm2, the current strength is 1.6 A. The electron concentration in the conductor is 1023 m ~ 3 at a temperature of 20 ° C. Find the average speed of the directed motion of electrons and compare it with the thermal speed of electrons.
3, For 4 s, the current strength in the conductor l "increased linearly from 1 to 5 A. Plot a graph of the current strength versus time. What charge has passed through the cross section of the conductor during this time?
Fredledikaskelinjj / Oct 28 2014 02:41:35
Determine the resistance of an aluminum wire 150 cm long if its cross-sectional area is 0.1 mm2. What is the voltage at the ends of this wire,if the current in it is 0.5 A?
In most new buildings, electrical wiring is initially made of copper wires. This is dictated by the increased load on the network, caused by a large number of electrical appliances. In addition, copper is more durable, does not oxidize and has best performance electrical conductivity.
But in old houses, aluminum wiring is everywhere. Many people planning overhaul, change aluminum wires to copper. However, not everyone has this opportunity. In addition, sometimes replacement is not possible for technical reasons.
What you should know
In these cases, it is necessary to connect aluminum and copper conductors to each other. But such a connection by simple twisting is prohibited: electrochemical corrosion begins between the wires, caused by natural humidity, such contact is rapidly destroyed. It is best to connect wires from the same material.
But the connection of copper and aluminum conductors is quite common. For this you can use various ways which have proven themselves in practice. The most used options for making such a connection are presented below.
Methods for reliable connection of different wires
There are several ways to connect aluminum and copper in electrical wiring. The main task of all these methods is to ensure the reliability and durability of the contact, while minimizing the possibility of electrochemical corrosion.
screw connection
Screw connection of aluminum and copper conductors wires is simple, while being reliable and durable. This option can be used if it is necessary to connect wires of different or large section. The essence and technology of this method is as follows:
- The ends of both wires are cleaned of insulation (approximately 30 mm);
- With the help of round-nose pliers, the ends are bent into a circle.
Then take the bolt right size and diameter. The assembly of the structure is carried out in the following order:
- A regular washer is put on the bolt;
- Circumference of the first conductor;
- Another puck;
- Ring of the second wire;
- One more puck;
- The design is clamped with a nut;
One of the advantages of this method is the ability to connect more than two wires. The maximum number of strands to be clamped is only limited by the length of the bolt.
When making such a connection, do not forget to put washers between the wires: copper should not be allowed to come into contact with aluminum conductors.
Wire twisting
This method is also widely used in practice, but requires a special approach. In order for the twisting of copper and aluminum conductors to be durable, and corrosion does not form between them, it is better to proceed as follows:
- The cores are stripped of insulation (at least 4 cm);
- Copper wire must be tinned with tin solder;
- After that, the usual twisting of current-carrying wires is carried out among themselves;
- To increase the protection of such a connection from moisture, it can be treated with a special heat-resistant varnish;
- After the varnish has dried, the twist is securely insulated and ready for use.
Twisting should be done in such a way that the cores are twisted together. Wrapping one wire around another is unacceptable!
Terminal blocks
The use of screw blocks is very popular and widely used in practice. This method has proven itself best in electrical panels, where there is a need to connect a large number wires. The pads are also used in junction boxes, providing collapsible contacts, which facilitates revision and repair if necessary.
Consider the order of work when choosing this method to connect copper and aluminum:
- As usual, the ends of the wires need to be stripped. The insulation is removed by about 0.5–1 cm;
- After that, the stripped ends are inserted into the terminals and clamped with screws with medium force so as not to break the cores.
Advice! Before clamping solid wires with screws, it is better to flatten them a little with a hammer or pliers. This is necessary to increase the contact area.
This method is applicable to both black plastic pads and terminals with thinner insulation made of white plastic. When asked which block is better, there is an opinion that white terminal blocks are less reliable (in mechanical design). Therefore, they are more often used as an adapter for connecting lamps, chandeliers and other low-power consumers.
Separately, we note that it is possible to hide the terminals under the plaster only if they are enclosed in a junction box.
Clamps and terminal blocks WAGO
More modern version pads equipped with clamp German manufacturer WAGO. These terminals are available in two types:
- One-piece pads have a cast, often transparent body. To fix the cores, it is enough to insert the cleaned ends of the wires into such a cap, the clamp will securely fix them. The disadvantage of this method is its one-time use: in order to redo the connections, you will need to bite off the old clamps;
- Detachable terminal blocks are free from this disadvantage. A special lever makes it easy to fix the wires, and if necessary, disassemble the connection, just lift it up, the clamps will open and the ends will come out of the terminal.
Using these clamps, you can make a multi-core (from 2 to 8) connection, and also use the terminal block as an adapter for a branch in the wiring. Another advantage of this method of connecting copper and aluminum is that there is no need for additional insulation of the contacts. The body of WAGO pads is fully insulated and reliable.
Permanent connections
Finally, consider another way how to connect copper with aluminum wires. This will require a special riveting tool. Now such devices are widely popular, and many masters already have them.
The technology of this method is similar to the method using a bolt and nut. Consider how using a riveting tool, you can make a reliable connection of electrical wires:
- After stripping the cores from insulation, the ends are folded into a small ring with round-nose pliers. It is important that the diameter be as small as possible so that the rivet does not dangle too freely;
- Then the structure is assembled in the same order as with the screw method: copper and aluminum conductors are put on the stud, a small washer is used as a gasket;
- After that, the rivet rod is placed in the head of the device, the handles of which are compressed until they click. Connection is ready!
The disadvantage of this method is the inability to disassemble the structure. If you need to connect another wire, the rivet will have to be cut out and reconnected. Also, do not forget about the importance of isolating this area: you can use cambric or insulating tape.
Summing up
We have studied the most common and used cores made of various materials: copper and aluminum. They are reliable, provide durable contact and exclude oxidation that leads to electrochemical corrosion.




